
| 4.0g |
| 5.0g |


 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������£�23g NO2����NA����ԭ�� | ||
| B��0.1mol?L-1���ռ���Һ�к���0.1NA��Na + | ||
| C��22.4 L��CO������1 mol N2�����ķ�������� | ||
D��9 g
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
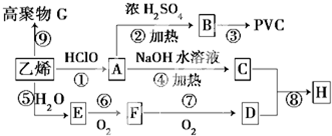
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
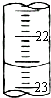 Ϊ�˲ⶨijNaOH��Ʒ��NaOH���������������ʲ����뷴Ӧ����ij��ѧ��ȤС�����������ʵ�飺
Ϊ�˲ⶨijNaOH��Ʒ��NaOH���������������ʲ����뷴Ӧ����ij��ѧ��ȤС�����������ʵ�飺| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.00 | 21.01 |
| 2 | 25.00 | 2.00 | 24.00 |
| 3 | 25.00 | 0.20 | 20.19 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��X��M��ԭ�����γ���������������Ŀ֮�ȶ�Ϊ1��2�����ӻ����� |
| B������W��Z��MԪ�ص��⻯����Է����������μ�С��������е����ν��� |
| C��Ԫ��Y��Z��W�ĵ��ʾ�������ͬ�����͵ľ��� |
| D��Ԫ��W��M���ж��ֵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������7 | B������7 |
| C��С��7 | D����ȷ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com