
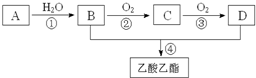
 ��֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��
��֪��A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·����ͼ��ʾ��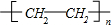 ���ʴ�Ϊ��
���ʴ�Ϊ��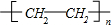 ��
�� CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O�� CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��| 4.48��108L |
| 22.4L/mol |
 CH3COOCH2CH3+H2O����֪�������Ҵ������ʵ���֮��Ϊ1��1����������������Ϊ2��107mol��
CH3COOCH2CH3+H2O����֪�������Ҵ������ʵ���֮��Ϊ1��1����������������Ϊ2��107mol��| 1 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ħ�������ʵ�������λ |
| B��������Ħ��������2g |
| C��1molNH3��������17g |
| D��1mol������ռ�����ԼΪ22.4L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ�ǰ | �ζ��� | |
| ��һ�� | 0.70 | 16.15 |
| �ڶ��� | 0.05 | 16.35 |
| ������ | 0.35 | 15.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| J | ||||
| R |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
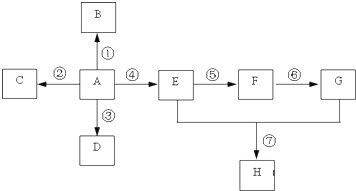
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com