
����Ŀ��G��ҩ��ϳ��е�һ����Ҫ�м��壬������G��һ�ֺϳ�·�ߣ�
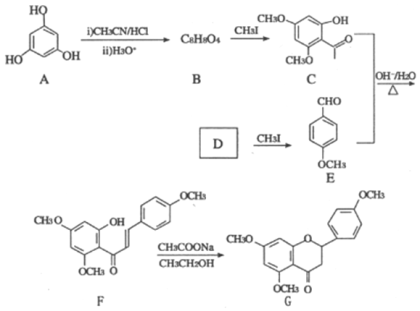
�ش��������⣺
��1��B�Ľṹ��ʽΪ__________���������������ŵ�����Ϊ__________��B����C�ķ�Ӧ����Ϊ__________��
��2��D��������__________��
��3����C��E�ϳ�F�Ļ�ѧ����ʽΪ________________________________________��
��4��D��ͬ���칹���У��ܷ���������Ӧ�ҷ��ӽṹ�к������Ļ���__________�֣����к˴Ź�����������6��壬�����֮��Ϊ1��1��1��1��1��1��ͬ���칹��Ľṹ��ʽΪ______________________ (һ�ּ���)��
��5�����������ϳ�·�ߣ���CH3CH2ClΪԭ��(�����Լ���ѡ)������Ʊ��Ͷ�ȩ(CH3CH=CH
CHO)�ĺϳ�·�ߡ�_______________
���𰸡�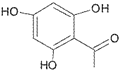 �ǻ����ʻ� ȡ����Ӧ ���ǻ�����ȩ
�ǻ����ʻ� ȡ����Ӧ ���ǻ�����ȩ 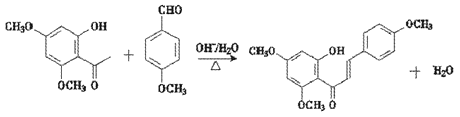 3
3 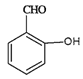 ��
��
![]()
��������
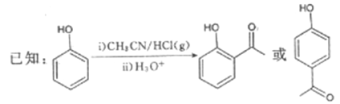
����A��C�ṹ������ʽ�����𣬽����֪�������ɵõ�B���ʵĽṹ��ʽ���京�еĹ����ŵ����ࣻ������B��C�ṹ�����������B����C�ķ�Ӧ���ͣ������ø÷�Ӧ�������Ʒ�����D�Ľṹ��ʽ������ϵͳ��������D���������ж�D��ͬ���칹��ʱ����ʱ��Ҫ�ӹ������칹��λ���칹���棬������Ҫ���������д����Ӧ�����ʵĽṹ��ʽ������C+E����Fʱ�ı仯������Ԫ�ص�ԭ���غ㣬�ɵø÷�Ӧ�Ļ�ѧ����ʽ���������֪����CH3CH2Cl�������Ŀ��֪��Ϣ���ϳ���Ҫ�Ʊ���Ŀ�����Ͷ�ȩ(CH3CH= CHCHO)��
(1)����A��C�ṹ����������֪��Ϣ��B�ķ���ʽ����֪B���ʵĽṹ��ʽ��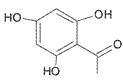 �������ʺ��еĹ��������ǻ����ʻ���B��CH3I����ȡ����Ӧ������C
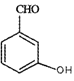
�������ʺ��еĹ��������ǻ����ʻ���B��CH3I����ȡ����Ӧ������C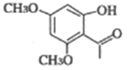 ��HI��
��HI��
(2)D��CH3I����ȡ����Ӧ�ɲ���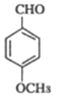 �����ƿ�֪DΪ���ǻ�����ȩ
�����ƿ�֪DΪ���ǻ�����ȩ![]() ��(3)��C
��(3)��C ��E
��E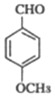 �ڼ��������¼��ȣ���Ӧ����F
�ڼ��������¼��ȣ���Ӧ����F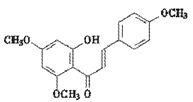 ��ˮ����Ӧ�Ļ�ѧ����ʽΪ
��ˮ����Ӧ�Ļ�ѧ����ʽΪ ��
��
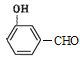
(4)D��![]() ���ж���ͬ���칹�壬���е�ͬ���칹����ӽṹ�к����������ܷ���������Ӧ˵������ȩ��������
���ж���ͬ���칹�壬���е�ͬ���칹����ӽṹ�к����������ܷ���������Ӧ˵������ȩ�������� ��
�� ��
��![]() ����3�֣����к˴Ź�����������6��壬�����֮��Ϊ1��1��1��1��1��1��ͬ���칹��Ľṹ��ʽΪ
����3�֣����к˴Ź�����������6��壬�����֮��Ϊ1��1��1��1��1��1��ͬ���칹��Ľṹ��ʽΪ ��
�� ��
��
(5)��CH3CH2ClΪԭ���Ʊ��Ͷ�ȩ(CH3CH= CHCHO)��������ˮ��õ��Ҵ����ٰ��Ҵ��������õ���ȩ�������������Ϣ�ϳɰͶ�ȩ������ϳ�·��Ϊ��![]() ��
��


 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A.����£�11.2 L���飨CH4������ϩ��C2H4���Ļ�������к���ԭ�ӵ����ʵ���Ϊ2 mol
B.����£�0.1 mol CCl4��ռ�����Ϊ2.24 L
C.��������N2��CO���еķ�������ԼΪ6.02��1023��
D.���³�ѹ�£�1 mol�κ�������ռ�������Ϊ22.4 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ҷ�������������( SaFeY)������ˮ����������ǿ�����У��ش��������⣺
ʵ��1 �Ʊ��Ҷ������������ƾ���
ʵ��ԭ����2Fe(OH)3+Na2CO3+2H4Y===2[NaFeY��3H2O]+CO2��+H2O
ʵ�鲽�裺�ٳ�ȡ2.7 g FeCl3��6H2O���ձ����ܽ⣬�����μ�������Ũ��ˮ�����裬���ˣ�ϴ�ӣ�����ڽ�Fe(OH)3���Ҷ���������(H4Y)��H2O����������ƿ(װ������ͼ)�����裬80��ˮԡ��Ӧ1h����Na2CO3��Һ����pH������һϵ�в���������ϴ�ӣ����ɵõ���Ʒ��

��1�����������Ϊ����Fe(OH)3�����й���������ʣ���ȡ�Ĵ�ʩ��___________��
��2���ж�����������г����Ƿ�ϴ�Ӹɾ��IJ���Ϊ___________��
��3������Һ©���滻Ϊ��Һ©����ʵ����Na2CO3��Һ����˳�����£���ԭ��Ϊ___________��
��4������������е���һϵ�в�����Ϊ___________(����)��������Һֱ�����ɣ������___________��
A.����Ũ�������Ƚᾧ
B.����Ũ������Һ������־�Ĥ��ֹͣ����
C����Ũ������������������ֹͣ����
ʵ��2������ǿ�������������ⶨ
��֪������ǿ���κ���NaCl��KIO3 ��NaFeY������n(KIO3)�Un( NaFeY)=1�U50
��I2+2S2O32��===2I��+ S4O62��
��ȡmg��Ʒ����ϡ�����ܽ�����100mL��Һ��ȡ��10mL�������Թ�����KI��Һ����ַ�Ӧ���������Һ����cmol��L��1Na2S2O3��Һ�ζ����ظ�����2~3�Σ�����Na2S2O3��Һƽ��ֵΪVmL��
��5��I��������Fe3+��Ӧ�⣬���ɷ����ķ�Ӧ�����ӷ���ʽΪ___________��
��6���ζ��յ������Ϊ___________����ɫ�仯)��
��7����Ʒ����Ԫ�ص���������Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵı仯�����У����ڷ��ȷ�Ӧ���ǣ� ��
A. ˮ������Ϊˮ�ų�����B. ϡ��Ũ����
C. ��ʯ����ˮ��ӦD. ����طֽ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڻ�ѧ��Դ��������������ǣ� ��
A. ��ѧ��Դ���Ǹ���ԭ��صĹ���ԭ����Ƶ�
B. �ڸɵ���У�̼��ֻ�����ã������μӻ�ѧ��Ӧ
C. ���ӵ�ز������ⶪ����ԭ���������ӵ���Դ���ޣ��۸�
D. ȼ�ϵ����һ�ָ�Ч�����������ͻ�ѧ��Դ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�������ֵ�����������������
��18g���ʯ�У�̼̼���ۼ���Ϊ3NA
��1molͭ��������������Ӧ��ת�Ƶĵ�����ΪNA
�۳��³�ѹ�£�S2��S6�Ļ���ﹲ6.4g������������ԭ����һ��Ϊ0.2NA
��һ���¶��£�1L 0��50mol L��1NH4Cl��Һ��2L 0.25molL-1NH4Cl��Һ�е�NH4+����Ŀ������0.5NA����ǰ�߸���
�ݵ�ⷨ����ͭʱ����������������64gʱ����·��ͨ���ĵ�����һ��Ϊ2NA
A. ��B. �ڢܢ�C. �ܢ�D. �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������һ�����Ϳɳ���أ��ܳ�ʱ�䱣���ȶ��ķŵ��ѹ�������ܷ�ӦΪ��3Zn + 2K2FeO4 + 8H2O![]() 3Zn(OH)2 + 2Fe(OH)3 + 4KOH��������������ȷ����
3Zn(OH)2 + 2Fe(OH)3 + 4KOH��������������ȷ����
A. �ŵ�ʱ������ӦΪ��Zn-2e-+2OH-= Zn(OH)2
B. ���ʱ��������������Ӧ��������Һ������ǿ
C. ���ʱÿת��3mol���ӣ�������1.5molZn����
D. �ŵ�ʱ������ӦΪ��FeO42- + 3e- + 4H2O = Fe(OH)3 + 5OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵����ȷ����( )
A.���³�ѹ�£�11.2 L���������е�ԭ����ĿΪNA
B.9 gˮ�����е���ԭ����ĿΪNA
C.��ͬ��ͬѹʱ, ��ͬ���ʵ������κ�����������ͬ��Ϊ11.2L
D.1 mol NH4+ ����������Ϊ10NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
ͭ�����ͽ����ճ������г��ý���
��1����̬ͭԭ�ӵļ۲�����Ų�ʽΪ___________.
��2��������Һ��Ҫ�ɷ���[Ag��NH3��2]OH�����Ʒ����ǣ���AgNO3��Һ�еμӰ�ˮ�������պ���ȫ�ܽ�Ϊֹ���õ������������Һ
��AgNO3�������ӵĿռ乹����_______________��
��[Ag��NH3��2]+�������ӵ���λ��Ϊ___________��NH3������N���ӻ�������__________��
����NH3��Ϊ�ȵ�����������У�_____________��
��3���ִ���ҵұ���У�2Au��CN��2��+Zn====2Au+Zn��CN��42����CN���dz��������壬�ṩ�µ��Ӷ���C����N������Ҫԭ����_________________________________��
��4��ͭ�������л���Ӧ�����Ĵ����� CH3CH2OH ![]() CH3CHO+H2����CH3CH2OH�ķе����CH3CHO����Ҫԭ����________����ԭ�ӹ���ص���ʽ���࣬H2����������������____________��
CH3CHO+H2����CH3CH2OH�ķе����CH3CHO����Ҫԭ����________����ԭ�ӹ���ص���ʽ���࣬H2����������������____________��
��5��һ��ͭ���Ͻ��׳ư�ͭ���ľ�����ͼ1��ʾ��ͭ����ԭ�Ӹ�����Ϊ___________��

��6������ͼ2��ʾ�����־���ѻ���ʽ��Ϊ___________�ѻ����þ�����ԭ�ӿռ������ʣ�![]() ��Ϊ___________ ����������ʽ�ӱ�ʾ��������ʾԭ�ӿռ�������=
��Ϊ___________ ����������ʽ�ӱ�ʾ��������ʾԭ�ӿռ�������=![]() ��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com