
��14�֣���֪��AΪ���������ӵĵ���ɫ���廯̨�E��XΪ�����г������壬A��B��C��D������ͬ�Ľ������ӣ���ת����ϵ����ͼ�����ֲ�������ȥ����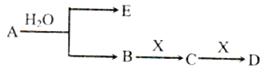
��ش���������
��1�����ֽ������ӵ����ӽṹʾ��ͼΪ_____________;
��2��X�ĵ���ʽ_______________;
��3��B��������ѧ����������_____________;
���³�ѹ�£�7.8gA��������ˮ��ַ�Ӧ�ų�����a kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ
_________________________________________________________________.
��4����CҲ��ת��ΪB��д����ת���Ļ�ѧ����ʽ_______________________________;
��B��D��Ӧ�����ӷ���ʽΪ_______________________________________.
��5����һ����������Xͨ��2LB����Һ�У���������Һ�б���μ���ϡ��������������� �������������������ʵ����Ĺ�ϵ��ͼ������������ܽ��HCl�Ļӷ�����
��ش�a����Һ���������ʵĻ�ѧʽΪ__________ ��b����Һ�и�����Ũ���ɴ�С�Ĺ�ϵ��_________________________________��
��14�֣�
��1��Na+ (1��)
(1��)
��2�� (2��)
(2��)
��3�������ԣ����ۼ������Ӽ���2�֣�2Na2O2(s)+2H2O(l)=4NaOH(aq)+ O2(g)����H=-20akJ/mol(2��)
��4����Ca(OH)2+Na2CO3=CaCO3��+2NaOH�� Ba(OH)2+Na2CO3=BaCO3��+2NaOH
(2��)
��OH-+HCO3-=CO32-+ H2O(2��)
��5��Na2CO3��NaCl (2��)
c(Na+)>c(Cl-)>c(HCO3-)> c(OH-)> c(H+)> c(OH-)> c(CO32-)( c(CO32-)��д�ɲ�д��������������)��2�֣�
���������������1��AΪ���������ӵĵ���ɫ���廯�����AΪNa2O2������������Na+�����ӽṹʾ��ͼΪ ��
��
��2��A��B��C��D������ͬ�Ľ������ӣ���BΪNaOH��CΪNa2CO3��DΪNaHCO3��XΪCO2�������ʽΪ ��
��
��3��NaOH�к������Ӽ����ۼ���
��4����Na2CO3ת��ΪNaOH���������ֽⷴӦ����Ҫ�Լ��������ƻ�������������ѧ����ʽΪCa(OH)2+Na2CO3=CaCO3��+2NaOH��Ba(OH)2+Na2CO3=BaCO3��+2NaOH��
��NaOH��NaHCO3��Ӧ����̼���ƺ�ˮ�����ӷ���ʽΪOH-+HCO3-=CO32-+ H2O��
��5����ͼ��֪����������̼������2������ᣬ����������̼֮ǰ��3������ᣬ��Na2CO3��NaHCO3��Ҫ��������NaHCO3��CO2��Ҫ�����������ͬ��˵���μ�����ǰ����Һ�е�����ΪNa2CO3��NaOH�������ʵ���֮��Ϊ2:1������a��֮ǰ�����ķ�Ӧ���������������Ƶ��кͷ�Ӧ��a�������ΪNa2CO3��NaCl��b��ʱNa2CO3ȫ��ת��ΪNaHCO3����ʱ����ΪNaHCO3��NaCl�������ʵ���֮��Ϊ2:3��������Һ�е�����Ũ�ȴ�С��ϵΪc(Na+)>c(Cl-)>c(HCO3-)> c(OH-)> c(H+)> c(OH-)> c(CO32-)��
���㣺�������ӽṹʾ��ͼ������ʽ����ѧ����ʽ�����ӷ���ʽ���ж�����д������Ũ�ȴ�С�ıȽ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��M��N������Һ�����ⶨ��������Һ�к�������12�����ӣ�Al3����Cl����Na����K����NO3-��OH����Fe2����AlO2-��CO32-��NH4+��SO42-��H����
(1)������б�����ʵ��ٵĽ��ۺ�ʵ��ڵ�ʵ�������Լ�����
| ʵ�������Լ����� | ���� |
| ��ȡ����N��Һ�μ����������ᱵ��Һ���������� | N�в��� ���� |
| �� | ȷ��M��Һ�к���Na��������K�� |
 ����pH��ֽ���M��Һ��pH��ֽ����ɫ ����pH��ֽ���M��Һ��pH��ֽ����ɫ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ��ˮ�г�����һ������Cr2O72����CrO42�������ǻ�����༰��̬ϵͳ�����ܴ���������д��������õĴ������������֡�����һ�ַ����ǻ�ԭ��������
�÷��Ĺ�������Ϊ��CrO42��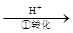 Cr2O72��
Cr2O72��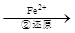 Cr3+
Cr3+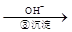 Cr(OH)3��
Cr(OH)3��
���еڢٲ�����ƽ�⣺2CrO42��(��ɫ)+2H��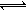 Cr2O72��(��ɫ)+H2O
Cr2O72��(��ɫ)+H2O
��1������ƽ���ǿ���Ի���������Һ��________ɫ��
��2����˵���ڢٲ���Ӧ��ƽ��״̬���� ��
A��Cr2O72����CrO42����Ũ����ͬ B��2v(Cr2O72��)=v(CrO42��) C����Һ����ɫ����
��3���ڢڲ��У���ԭ1molCr2O72�����ӣ���Ҫ mol��FeSO4��7H2O��
д���ڢڲ��з�Ӧ�����ӷ���ʽ_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ȣ�ClO2��Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
��1����ҵ���Ʊ�ClO2�ķ�Ӧԭ�������ã�2NaClO3��4HCl=2ClO2����Cl2����2H2O��2NaCl��
�� Ũ�����ڷ�Ӧ����ʾ������������_______������ţ���
| A��ֻ�л�ԭ�� | B����ԭ�Ժ����� | C��ֻ�������� | D�������Ժ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪ij��ɫ��Һ�п��ܺ���Na+��Ba2+��Fe3+��MnO4����SO32����Cl����HCO3�� �еļ��֣����ν�������ʵ�飬�۲쵽���������£�����PH��ֽ���飬��Һ��PH��7��
������Һ�еμ���ˮ��û������������õ���ɫ��Һ��
����ڵ���Һ�еμ������ữ��������Һ��������ɫ������
����ڵ���Һ�еμ�BaCl2��Һ����������������İ�ɫ������
��1��ԭ��Һ��һ�����е��������� ���϶�û�е��������� ��
��2������ڵ����ӷ�Ӧ����ʽΪ ��
��3���ܷ�ȷ������������ �������У�д�����ӷ��ţ�û����дû�У����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ˮ��������ʱ������˫����(H2Dz����Ԫ����)�ѽ���������ϳɵ����Ե����ʣ�����CCl4��ȡ�����Ӷ��ѽ������Ӵ�ˮ��Һ����ȫ�������������˫����(H2Dz)��CCl4������ˮ�е�Cu2+ʱ���ȷ�����Ϸ�Ӧ��Cu2++2H2DZ Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
Cu (HDZ)2+2H+���ټ���CCl4��Cu (HDZ)2�ͺ����ױ���ȡ��CCl4�С�
��1��д��˫�����Fe3+��ϵ����ӷ���ʽ��_____________________����ȡFe3+�Ĺ�����Ҫ�������˵���ȣ������Һ��pH����������___________________________��
��2����ͼ����˫����(HzDz)��CCl4�����ȡijЩ�������ӵ�������ߣ�����ӳ����ȡijЩ��������ʱ���˵�pH��Χ��E����ʾij�ֽ����������������ʽ��ȡ����İٷ��ʡ�
ij��ҵ��ˮ�к���Hg2+��Bi3+��Zn2+����˫���꣨H2Dz��~ CCl4�����ȡ��������ˮ��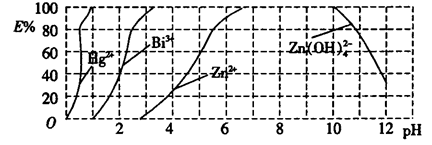
������ȫ����ˮ�е�Hg2+����������������Һ��pH=________��
�ڵ�����pH=2ʱ����(Bi)�Ĵ�����ʽ��_________________��
��3����ˮ�е��ǹ�����(Hg2+ 2)����ת���ɹ�����(Hg2+)������˫������ϡ�ij������ˮ�к��н϶���Ȼ��ǹ�(Hg2Cl2)������������(K2S2O8)������(Hg2+ 2)��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ���֪��
�����ԣ�IO3-��Fe3����I2����ԭ�ԣ�S2O32-��I��
3I2��6OH��=IO3-��5I����3H2O��KI��I2 KI3
KI3
(1)ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ټ�KSCN��Һ���Ժ�ɫ���ú�ɫ������_____________(�û�ѧʽ��ʾ)��CCl4�����Ϻ�ɫ��������___________(�õ���ʽ��ʾ)��
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ_______________________________________��__________________________________________________________________��
(2)KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��
д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ��_____________________��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O����������Ϊʳ�μӵ���Ƿ���ʣ�___________ (��ǡ���)����˵������_________________________________________________��
(3)Ϊ����ӵ���(����KI)���ȶ��ԣ��ɼ��ȶ������ٵ����ʧ�������������п�����Ϊ�ȶ�������_______��
| A��Na2S2O3 | B��AlCl3 | C��Na2CO3 | D��NaNO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ijԪ���ڻ�ѧ��Ӧ���ɻ���̬��Ϊ����̬�����Ԫ��
| A��һ���������ˡ������� | B��һ������ԭ�� |
| C���ȿ��ܱ���ԭҲ���ܱ����� | D���Ȳ����ܱ������ֲ����ܱ���ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ɫ��ѧ���������Ի�������Ⱦ�����������п��Գ�Ϊ����ɫ������������
| A��Ũ���� | B������ | C��˫��ˮ | D��Ư�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com