
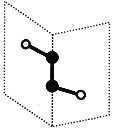
����Ŀ������������Ԫ��X��Y��Z��W��ԭ���������ε���������Y��Zͬ���塣X��Y��Z��W�����γ���ͼ��ʾ�ķ��ӽṹ����Z��W�γɵķ���������ԭ������������8�����ȶ��ṹ������˵������ȷ���ǣ� ��
A.���Ӱ뾶��W>Z>Y>X
B.ZY2��WY2������ʹƷ����Һ��ɫ����ɫԭ����ͬ
C.�������Ӧ��ˮ��������ԣ�W>Z
D.X2Y2��Z2W2�����зǼ��Թ��ۼ��Ҹ�ԭ�Ӷ�����8���ӵ��ȶ��ṹ
���𰸡�B
��������
����������Ԫ��X��Y��Z��W��ԭ���������ε�����X��Y��Z��W�����γ���ͼ��ʾ�ķ��ӽṹ����Z��W�γɵķ���������ԭ������������8�����ȶ��ṹ�����ͼʾ��ZΪS��WΪClԪ�أ�����Y��Zͬ���壬��YΪOԪ�أ�X��Y�γɵĸû�����Ϊ˫��ˮ����XΪHԪ�ء�
���ݷ�����֪��XΪH��YΪO��ZΪS��WΪClԪ�ء�
A��ͬһ���ڴ������������Ӱ뾶��С��S2��>Cl����ͬһ������ϵ��������Ӱ뾶������S2��>O2������A����
B��SO2��ClO2������ʹƷ����Һ��ɫ����ɫԭ����ͬ��ǰ�����ɲ��ȶ�����ɫ���ʣ����߷���������ԭ��Ӧ����B��ȷ��
C��Ӧ������������Ӧ��ˮ��������ԣ�W>Z����C����
D��H2O2��H������8���ӵ��ȶ��ṹ����D����
��ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ȡ��ԭ�����ؽṹ����ͼ��ʾ������������ԣ��翹���������ɻ���������ȣ��ɷ�ֹ������֬�����������ɻ��IJ����������������ȶ��ּ������й�ԭ�����ص�����˵������ȷ����(����)

A. �����ʼȿɿ������࣬Ҳ�ɿ�������
B. 1 mol�����ʿ���4 mol Br2��Ӧ
C. 1 mol�����ʿ���7 mol NaOH��Ӧ
D. 1 mol�����ʿ���7 mol Na��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͷ������֡��л��������߿Ƽ��������й㷺Ӧ�á�һ�ִ�����ҵ�ķϴ�������Ҫ�ɷ�ΪV2O5��MoO3��Al2O3�������̼�⻯����л����⡢���Ĺ���������ͼ��
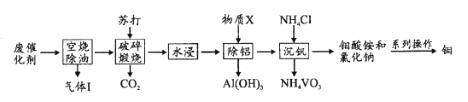
��1������������CO2��H2O�⣬�����е���Ҫ�ɷ���____��
��2������������Ŀ����____��������V2O5ת���Ļ�ѧ����ʽ��____��
��3����������X��Ŀ���ǵ�����ҺpH������X�Ļ�ѧʽ��____��
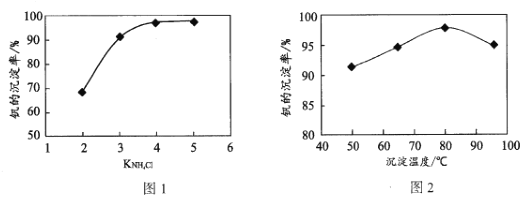
��4���������������п���pHΪ8.35����������һ��ʱ�����ij���������ϵ��KNH4Cl���¶ȵĹ�ϵ�ֱ���ͼ1��ͼ2����Ӧѡ��ļ��ϵ����____���¶ȳ���80��Cʱ�����ij������½���ԭ����____��
��5�������[��NH4��2MoO4]�ڸ�����ͨ��H2���Ƶõ����⣬�ù��̵Ļ�ѧ����ʽ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO2�����ת��Ϊ�߸���ֵ��CO��CH4��CH3OH��C1����ù��̿ɻ���CO2�����Ļ���ѹ����ͬʱ�ɱ��Ϊ����������ľ���Ч�档CO2������̣���Ҫ����������������ӦΪ��
��Ӧi��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��H=-49.01kJmol-1
CH3OH(g)+H2O(g)��H=-49.01kJmol-1
��Ӧii��CO2(g)+4H2(g)![]() CH4(g)+2H2O(g)��H=-165.0kJmol-1
CH4(g)+2H2O(g)��H=-165.0kJmol-1
��Ӧiii��CO2(g)+H2(g)![]() CO(g)+H2O(g)��H=+41.17kJmol-1
CO(g)+H2O(g)��H=+41.17kJmol-1
�ش��������⣺
��1����CO��H2�ϳɼ״����Ȼ�ѧ����ʽΪ_____��
��2����ӦiiiΪ��ˮú���任��Ӧ�����RWGS���Խ�������Ϊ�����÷�Ӧ���̵���ʾ����������(eV)�仯ͼ��ͼ��ʾ(![]() Ϊ������
Ϊ������![]() ΪCԭ�ӣ�
ΪCԭ�ӣ�![]() ΪOԭ�ӣ�oΪHԭ�ӣ�
ΪOԭ�ӣ�oΪHԭ�ӣ�
����I��

����II��
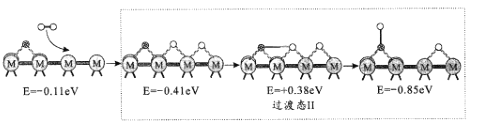
����III��
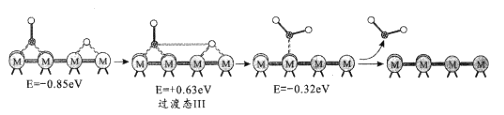
������1�����ڷ�Ӧ�ķ���ʽΪCO2*+*=CO*+O*(*Ϊ��������λ�㣩������ͼʾ���䷴Ӧ����H_____0(������������<��)��
�ڷ�Ӧ����II�����ڵķ���ʽ��_____��
�۷�Ӧ������_____����������I����������II����������III������RWGS�Ŀ��ٲ��衣
��3���ҹ���ѧ���о��˲�ͬ��Ӧ�¶ȶԺ�̼����ɵ�Ӱ�졣�ڷ�Ӧ���а� =3��1ͨ��H2��CO2���ֱ���0.1MPa��1MPa�½��з�Ӧ���������¶ȶ�ƽ�����C1(CO2��CO��CH4)�е�CO��CH4��Ӱ����ͼ��ʾ���÷�Ӧ�����¼״��������ͣ���˺�������Ӧi��)��
=3��1ͨ��H2��CO2���ֱ���0.1MPa��1MPa�½��з�Ӧ���������¶ȶ�ƽ�����C1(CO2��CO��CH4)�е�CO��CH4��Ӱ����ͼ��ʾ���÷�Ӧ�����¼״��������ͣ���˺�������Ӧi��)��

��1MPaʱ����ʾCH4��COƽ��������¶ȱ仯��ϵ�����߷ֱ���_____��_____��M��COƽ����ɺ�������N���ԭ����_____��
�ڵ�CH4��COƽ�����Ϊ40%ʱ�����¶��·�Ӧiii��ƽ�ⳣ��KpΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ǻϳ�ҩ����м��壬һ�ֺϳ������·����ͼ��

��1��A��B������Լ�Ϊ_____��
��2��������B��һ������������C���Ҵ���������B�ϼ���λ��Ϊ_____������ţ�
��3��C��D�ķ�Ӧ����Ϊ_____��D��E�ķ�Ӧ����ʽ��_____��
��4��������G�У����ǰ�������еĹ�����������_____��
��5��F��ͬ���칹��W����̼��������Һ��Ӧ�ų����壬����ʹ��ˮ��ɫ�����к˴Ź������������Ϊ6��1��1��W�Ľṹ��ʽΪ_____��
��6����������·�ߣ�����Ա�����![]() ����
���� �Ʊ���
�Ʊ��� �ϳ�·�ߣ����Լ���ѡ��_____��
�ϳ�·�ߣ����Լ���ѡ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʵ��������������ʵ���Ũ�ȵļ������������
A.�ܶ�Ϊ0.91g/cm3�İ�ˮ����������Ϊ25%���ð�ˮ�õ������ˮϡ�ͺ�������Һ�����ʵ�������������12.5%
B.��K2SO4��Al2(SO4)3�Ļ����Һ����֪����Al3�������ʵ���Ũ��Ϊ0.4 mol/L ��SO42�������ʵ���Ũ��Ϊ0.7 mol/L �������Һ��K�������ʵ���Ũ��Ϊ0.2 mol/L
C.��5 mol/L ��Mg(NO3)2��ҺamL ϡ����bmL��ϡ�ͺ���Һ��NO3�������ʵ���Ũ��Ϊ![]() mol/L
mol/L
D.����״���£���VL A���壨Ħ������ΪMg/mol������0.1Lˮ�У�������Һ�ܶȦ�g/cm3�������Һ�����ʵ���Ũ��Ϊ![]() mol/L
mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ����Ҫ�Ļ���ԭ�ϣ��ڹ�ũҵ������������������Ҫ��Ӧ�á�ʵ������ȡ����������Ũ����Ͷ�������������������з�Ӧ��KClO3+6HCl(Ũ)=KCl+3Cl2��+3H2O,�÷�Ӧ���ŵ��Ƿ�Ӧ���������ٶȿ졢����Ҫ���ȡ����������ѧ֪ʶ�ش���������
(1)�÷�Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ_____________________��
(2)������ͨ����ɫʯ����Һ�У����Թ۲쵽��������________________________________��
(3)ʵ�������ƺ�2.0mol/L NaOH��Һ����Ҫ������Һ____________mL������4.48L��������״����ǡ����ȫ��Ӧ��
(4)��֪Br2��ˮ��Һ��Ũ�Ȳ�ͬ�����ֳ�ɫ�����ɫ��NaBr��Һ�л���ͨ��Cl2ʱ�����Կ�����ɫ��Һ��Ϊ����ɫ����д����Ӧ�����ӷ���ʽ_________________________________��
(5)����490mL 2.0mol/L NaOH��Һ��
������������������ƹ����������__________________��
������ʵ����Ҫ����������ƽ�������룩��ҩ�ס��ձ�����Ͳ������������ͷ�ιܡ�______________��
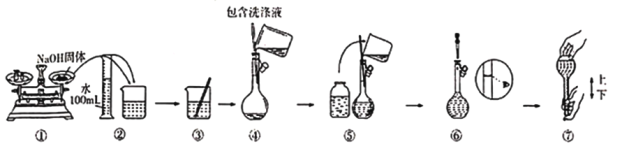
��ijͬѧ�������ƹ�����ͼ��ʾ������Ϊ�д���IJ�����_____________________������������ʾ����ʱ��ָ������ƫ����������ҺŨ�Ƚ�____________________���ƫ�ߡ���ƫ�͡����䡱��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ͼװ�ý���ʵ��̽������ش��������⣺

��1������ת��Ϊ��ѧ�ܵ�װ��Ϊ_______�أ�����A������B������
��2��Aװ��пΪ_______����ʵ�������������Ũ�Ƚϴ��������______������Zn�缫������Cu�缫������
��3��Bװ��ʯī2Ϊ_______�����缫��ӦʽΪ_______����ʯī1�ų�2240mL���壨��״���£�ʱ����·��ת�Ƶ��ӵ���ĿΪ_________����Aװ��Ҳת����ͬ�����ĵ��ӣ�п������������_______g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ��ͬ��ѧԪ����ɵĻ�����ʾ��ͼ������˵����ȷ���ǣ� ��
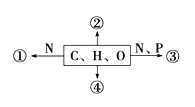
A. ��ͼ�Т�Ϊij�ֻ�����Ļ�����λ�����������Ǻ�����
B. ���ڹ㷺�ֲ��ڶ���ϸ���ڣ�����һ������ԭ
C. ����Ϊ�������ӣ����䳹��ˮ��������Ϊ4��
D. ����Ϊ��Ҫ�������ʣ������ֲ��ϸ����������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com