
����Ŀ����п��ZnS����һ����Ҫ�Ļ���ԭ�ϣ�������ˮ��������п�ķ���п����ȡ�乤��������ͼ��ʾ��

��1��Ϊ���п�ҵĽ�ȡ�ʣ��ɲ��õķ�����______________������ţ���
����ĥ �ڶ�ν�ȡ �������¶� �ܼ�ѹ �ݽ���
��2����������������е������ǣ�д��ѧʽ��_____________��
��3��������пɵ�Cd���ʣ�Ϊ���������µ����ʣ��Լ�XӦΪ________��
��4������������Ի���Na2SO4����ȡNa2S��
�ټ���ZnS�����Ƿ�ϴ�Ӹɾ��ķ�����__________________��
��Na2S���ɵ����ʵ�����Na2SO4��CH4�ڸ��¡�������������ȡ����ѧ��Ӧ����ʽΪ_______��
����֪Na2SO4��10H2O��Na2SO4���ܽ�����¶ȱ仯������ͼ������Һ�еõ�Na2SO4��10H2O�IJ���������_________________________________��

��5�������������ZnCO3Ϊbmol�����������CdΪdmol�����õ�VL�����ʵ���Ũ��Ϊcmol/L��Na2SO4��Һ��������������п���к���пԪ�ص�����Ϊ__________��
���𰸡� �٢ڢۢ� Fe(OH)3 Zn ȡ�������һ��ϴ��Һ���Թ��У��ȼ���HCl�ữ���ټ����Ȼ��������ް�ɫ�������ɣ�����ϴ�� Na2SO4+CH4![]() Na2S+CO2+2H2O ����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����� 65(Vc-b-d)
Na2S+CO2+2H2O ����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����� 65(Vc-b-d)
�������������������������п�ķ���п���Ʊ�ZnS�Ĺ�������Ϊ���壬�������̵ķ���������ʵ���������ѧ���㡣
��1������I��п���м���ϡ����õ���ȡҺ����ȡҺ�к�Zn2+��Cd2+��Fe3+��Fe2+�����٣���ĥ������п��������Һ�ĽӴ��������߽�ȡ�ʣ��ڣ���ν�ȡ��߽�ȡ�ʣ��ۣ������¶ȼӿ��ȡ���ʣ���߽�ȡ�ʣ��ܣ���ȡ����Ϊ�����Һ��ķ�Ӧ��û��������룬��ѹ������߽�ȡ�ʣ��������������п��������Һ�ĽӴ��������߽�ȡ�ʣ���߽�ȡ�ʿɲ��õķ������٢ڢۢݣ���ѡ�٢ڢۢݡ�
��2������II��п�ҽ�ȡҺ�м���H2O2��H2O2��Fe2+������Fe3+��Ȼ���������ZnCO3������Һ��pH��ʹFe3+ת����Fe��OH��3ͨ�����˵ķ�����ȥ������II���������е����ʵĻ�ѧʽΪFe��OH��3��
��3����ҺI�к�Zn2+��Cd2+������III�пɵõ�Cd���ʣ�Ϊ�������������ʣ��Լ�XӦΪZn�������ķ�ӦΪZn+Cd2+=Zn2++Cd��
��4������IVΪ��ZnSO4��Һ�м���Na2S����ZnS��Na2SO4����Ӧ�Ļ�ѧ����ʽΪNa2S+ZnSO4=ZnS��+Na2SO4��
������ZnS�����Ƿ�ϴ�Ӹɾ�����������ϴ��Һ���Ƿ�SO42-���ɣ�ʵ�鷽���ǣ�ȡ�������һ��ϴ��Һ���Թ��У��ȼ���HCl�ữ���ټ���BaCl2�����ް�ɫ�������ɣ�����ϴ����
�������ʵ�����Na2SO4��CH4�ڸ��¡����������·�Ӧ����Na2S������ԭ���غ㻹����CO2��H2O����Ӧ�Ļ�ѧ����ʽΪNa2SO4+CH4![]() Na2S+CO2+2H2O��
Na2S+CO2+2H2O��
�������ܽ�����ߣ�����Һ�еõ�Na2SO4��10H2O�IJ��������ǣ�����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����
��5������II�м���bmolZnCO3��ȥFe3+����Һ������bmolZn2+������III��Zn��Cd2+�����û���Ӧ��Zn+Cd2+=Zn2++Cd���õ�dmolCd��ͬʱ��Һ������dmolZn2+������IV�еķ�ӦΪNa2S+ZnSO4=ZnS��+Na2SO4����ҺII��n��Zn2+����=n��Na2SO4��=cVmol������Zn�غ���������п���к���пԪ�ص����ʵ���Ϊ��cV-b-d��mol��п���к���пԪ�ص�����Ϊ65��cV-b-d��g��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ���������ض��dz�����ˮ��������ij��ȤС������мΪԭ��ģ�ҵ���Ʊ��Ȼ�������һ�������Ʊ�������ص���������:

��ش���������:
(1)����������ͨ����������������������������е��ŵ���__________________________________��
(2)���ϳ���������Na2FeO4�����ӷ���ʽΪ__________________________________��
(3)Ϊ�˼���������������������FeCl3��Һ���Ƿ���Fe2+��ijͬѧȡ������Һ���Թ��У�ѡ�������Լ����ԴﵽĿ�ĵ���_______(����ĸ)��
a.KSCN��Һ b.NaOH��Һ c.K3[Fe(CN)6]��Һ d.������Һ
��ѡ������K MnO4��Һ���м��飬����������Ƿ��������˵������:_____________________________��
(4)��������ȡFeCl3����ľ������������_____________________________________________________����ʹ6.4mol/LFeCl3������Һ������Fe(OH)3���������Һ��pHС��_______{��֪��ʵ����������Ksp[(Fe(OH)3]=8.5��10-36��Kw=1.1��10-13��1.13��1.33}
(5)�ڲ�ͬ�¶ȺͲ�ͬpH�£�FeO42-���ȶ�������ͼ��ʾ:

����ͼ������Ϊ��������ϳ�����Na2FeO4�IJ��ʣ��ɲ�ȡ��ʵ��������ΧΪ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ơ��ȼ��仯����������ת����ϵ���밴Ҫ����գ�

��1��Na��Na2O��Na2O2��NaOH���ÿ��������ն��DZ�Ϊ____________���ѧʽ����
��2��Na����ʯ�����ϼ��ȷ�Ӧ�Ļ�ѧ����ʽΪ______________________________�� ��
��3��Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________________________________ ��
��4��һС�������Ͷ��CuCl2��Һ�У�������Ӧ�ķ���ʽ��:___________,��_________________________________________________________________.
��5���ڵ�ȼ��������Fe ��Cl2������Ӧ������Ϊ_____________________________________________����Ӧ�Ļ�ѧ����ʽΪ______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.��ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺
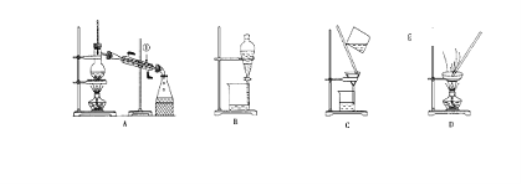
��1�����Ȼ�����Һ�еõ��Ȼ��ع��壬ѡ��װ��____________(�����װ��ͼ����ĸ����ͬ)����ȥ����ˮ�е�Cl�������ʣ�ѡ��װ��____________��
��2���ӵ�ˮ�з����I2��ѡ��װ��_________���÷��뷽��������Ϊ___________��
��3��װ��A�Тٵ�ˮ�Ľ���������_________��A�����в������������Ʒֱ���__________��
��.���ڻ����ķ�����ᴿ�������õķ����У�A����Һ B������ C����ȡ D������ E���ᾧ F�����ȷֽ⣬���и������ʵķ�����ᴿ��Ӧѡ��������������һ�֣�������ĸ��ţ�
��4����ȥCa(OH)2��Һ��������CaCO3__________��
��5������ֲ���ͺ�ˮ__________��
��6����ȥNaCl������������KNO3___________��
��7����ȥCaO������CaCO3__________��
��8����ʳ�þƾ������в�ҩ��ȡ���е���Ч�ɷ�_________��
��9�����յ��CCl4��Һ�е�CCl4__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̼��ƹ㷺Ӧ���������ϡ���ֽ����ѧ���ġ���ī��Ϳ�ϡ��ܷ⽺����ҵ����CaCl2��Һ��ͨ��NH3��CO2�����Ƶ�����̼��ơ�ij��ѧ��ȤС�������ͼ��ʾװ����ȡ�ò�Ʒ��D��װ��պ��ϡ�������֬�ޣ�ͼ�мг�װ������ȥ��
��ѡ�õ�ҩƷ�У�a��ʯ��ʯ b�������Ȼ�����Һ c.6mol/L���� d���Ȼ��e����������

��1��A���Ʊ�����ʱ������ҩƷ��______��ѡ����ĸ��ţ���
��2��B��ʢ��_____________��Һ����������___________________________��
��3��д����ʵ������ȡ�����Ļ�ѧ����ʽ________________________________��
��4����ʵ���У���C��ͨ�����������Ⱥ�˳��ģ�Ӧ��ͨ���������_____���ѧʽ����
��5������D���ڴ��а����ݳ��ķ�����_____________________________��
��6��д��������̼��ƵĻ�ѧ����ʽ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��RΪǰ������ԭ���������������Ԫ�ء�Xԭ����3���ܼ�����ÿ���ܼ��ϵĵ�������ȣ�Zԭ�ӵIJ��ɶԵ�������ͬ���������,��Z����̬�⻯����ͬ����Ԫ�ص��⻯���зе���ͣ�X��Y��R��Ԫ�������ڱ���ͬ�塣
��1��RԪ�������ڱ��е�λ����_______________________�����̬ԭ�ӵļ۲�����Ų�ͼΪ__________________________��
��2����ͼ��ʾX��Y��Z���ļ������ܱ仯���ƣ����б�ʾZ��������_________�����ţ���

��3�������XH2��X��O��������Xԭ���ӻ����������___________��1mol (X2H5O)3Z��O�����к��еĦҼ���м�����Ŀ��Ϊ_______________��
��4��Z��������Ӧ������һ�ָ�ԭ�Ӿ�����8�����ȶ��ṹ�Ļ��������ӵĿռ乹��Ϊ______��
��5��ijR�������ᄃ���ṹ����ͼ��ʾ�������ʵĻ�ѧʽΪ____________����֪�þ����ܶ�Ϊ��g/cm3���������������ԭ�ӵľ���Ϊd pm,��R�����ԭ������Ϊ___________________�� (�����ӵ�����ΪNA)

��6��X�γɵ�һ�ֳ������ʣ�����Ӳ���࣬ԭ����____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�����ŷŵĹ�ҵ��ˮ�к�Fe3+��Al3+��Cu2+������Ϊ�˷�ֹ������Ⱦ�����Ϊ�������÷���м��������ѧ�Լ��������²������õ����̷���FeSO4��7H2O����Al2O3�ͽ���Cu���ش��������⣺

��1������C��_________���û�ѧʽ��ʾ����
��2���ڹ��̢�����Ҫͨ���������������ù����з�����Ӧ�����ӷ���ʽΪ__________��
��3����ҺE��ɫ��Ӧ�ʻ�ɫ����Һa��______________��
��4������F����ҺH�������Լ�����_________,��____________����ҺG����ҺH��Ӧ�����ӷ���ʽ��_______________________��
��5��ʵ����������ҺH�õ��̷��IJ����ǣ�������Ũ��___________�����ˡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(ClNO)���л���ϳ��е���Ҫ�Լ������ɫҺ����ɫ���壬���д̱Ƕ��ζ����ˮ��Ӧ����һ���⻯������������ijѧϰС����ʵ������Cl2��NO�Ʊ�ClNO���ⶨ�䴿�ȣ����ʵ��(�г�װ����ȥ) ���¡���ش�:

��1���Ʊ�Cl2����װ�ÿ���_____(���д��ĸ)����Ӧ�����ӷ���ʽΪ____________________��
��2�����ռ�һƿ�����������ѡ��װ�ã�������˳��Ϊ:a![]() ______________________(����������,��Сд��ĸ��ʾ)�����õ�F����ʢװҩƷΪ____________________��
______________________(����������,��Сд��ĸ��ʾ)�����õ�F����ʢװҩƷΪ____________________��
��3��ʵ���ҿ�����ͼװ���Ʊ���������(ClNO);

��ʵ����Ҳ����Bװ���Ʊ�NO��Xװ�õ��ŵ�Ϊ________________________________��
�ڼ���װ�������Բ�װ��ҩƷ����k2��Ȼ���ٴ�________(����k1��"����k3��)��ͨ��һ��ʱ�����壬����Ŀ��Ϊ_________________________________________________����Ȼ�����������������Z��һ����Һ������ʱ��ֹͣʵ�顣
������װ��Y����Z��ClNO���ܷ�����Ӧ�Ļ�ѧ����ʽΪ__________________��
��4��ȡZ������Һ��m g����ˮ�����Ƴ�250mL��Һ��ȡ��25.00mL����K2CrO4��ҺΪָʾ������c mol��L��1AgNO3����Һ�ζ����յ㣬���ı���Һ�����Ϊ22.50mL��(��֪:Ag2CrO4Ϊש��ɫ����;
Ksp(AgCl)=1.56��10-10��Ksp(Ag2CrO4)=1��10-12)������������(ClNO)����������Ϊ_______________(�ô���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʢ��ϡ������ձ��з����õ������ӵ�пƬ��ͭƬ������������ȷ����(����)
A. ͭƬ�Ϸ����˻�ԭ��Ӧ
B. ����ͨ��������ͭƬ����пƬ
C. ������O2�ݳ�
D. ����������SO42-Ũ��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com