
����Ŀ��A��B��D��E��F��G��ԭ������������������ֶ�����Ԫ�ء�A��B���γ�B2A��B2A2���ֻ����B��D��G�����������Ӧˮ��������֮�䶼�ܷ�Ӧ��D��F��Gԭ������������֮�͵���15���ش��������⣺
(1)EԪ����Ԫ�����ڱ��е�λ����__________��A���ӵĽṹʾ��ͼΪ____________��
(2)D�ĵ�����B������������Ӧˮ�������Һ��Ӧ�������ӷ���ʽΪ__________��
(3)��B2A2�к���___________����_________���������ʽΪ__________��
�ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ_______________��
(4)����˵����ȷ����__________(����ĸ���)��
��B��D��Eԭ�Ӱ뾶���μ�С
������Ԫ�ص���������ϼ۾�������ԭ�ӵ�����������
��D������������Ӧˮ����������ڰ�ˮ
��Ԫ����̬�⻯����ȶ��ԣ�F>A>G
(5)��E��F��G������������Ӧˮ�����У�������ǿ��Ϊ_________(�ѧʽ)����ԭ�ӽṹ����ԭ��ͬ����Ԫ�ص��Ӳ�����ͬ���������ң�_________���õ�����������ǿ��Ԫ�طǽ���������ǿ��
���𰸡��������ڵڢ�A�� ![]() 2A1+2OH-+2H2O=2AlO2-+3H2�� ���� �Ǽ��Թ���
2A1+2OH-+2H2O=2AlO2-+3H2�� ���� �Ǽ��Թ��� ![]() 2Na2O2+2H2O=O2��+4NaOH �� HClO4 �˵���������࣬ԭ�Ӱ뾶��С
2Na2O2+2H2O=O2��+4NaOH �� HClO4 �˵���������࣬ԭ�Ӱ뾶��С
��������
A��B��D��E��F��G��ԭ������������������ֶ�����Ԫ�ء�B��D��G������������Ӧˮ��������֮�䶼�ܷ�Ӧ��������������ǿ�ǿ��֮��ķ�Ӧ����֪BΪNa��DΪAl��D��F��G ԭ������������֮�͵���15����F��G������������֮��Ϊ15-3=12����FΪP��GΪCl����֪EΪSi��A��B�ܹ��γ�B2A��B2A2 ���ֻ������AΪOԪ�ء�������ʽṹ��Ԫ�������ɷ������
��������������AΪOԪ�أ�BΪNaԪ�أ�DΪAlԪ�أ�EΪSiԪ�أ�FΪPԪ�أ�GΪClԪ�ء�
(1)EΪSi��λ�����ڱ��е������� �ڢ�A�壬AΪOԪ�أ������ӵĽṹʾ��ͼΪ![]() ���ʴ�Ϊ���������� �ڢ�A�壻
���ʴ�Ϊ���������� �ڢ�A�壻![]() ��
��
(2)B������������Ӧˮ����ΪNaOH��Al��NaOH��Һ��Ӧ���ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
(3)��Na2O2Ϊ���ӻ�������к������Ӽ��ͷǼ��Թ��ۼ�������ʽΪ![]() ���ʴ�Ϊ�����ӣ�����(��Ǽ��Թ���)��
���ʴ�Ϊ�����ӣ�����(��Ǽ��Թ���)��![]() ��
��
�ڹ���������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��2Na2O2+2H2O=O2��+4NaOH���ʴ�Ϊ��2Na2O2+2H2O=O2��+4NaOH��
(4)��ͬ���ڴ�������ԭ�Ӱ뾶��С����B(Na)��D(Al)��E(Si) ԭ�Ӱ뾶���μ�С���ʢ���ȷ����O����ۣ�ֻ��5��Ԫ�ص���������ϼ۾�������ԭ�ӵ��������������ʢڴ���D������������Ӧˮ����Ϊ��������������ǿ�ᡢǿ��������ڰ�ˮ���ʢ۴��ܷǽ�����Խǿ����Ӧ�⻯��Խ�ȶ�����Ԫ����̬�⻯����ȶ���A(��)��G(��)��F(��)���ʢܴ��ʴ�Ϊ���٣�
(5)��E��F��G������������Ӧˮ�����У�������ǿ��ΪHClO4����Ϊͬ����Ԫ�صĵ��Ӳ�����ͬ�������ң��˵���������࣬ԭ�Ӱ뾶��С���õ�����������ǿ��Ԫ�طǽ���������ǿ���ʴ�Ϊ��HClO4�� �˵���������࣬ԭ�Ӱ뾶��С��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ��������ȷ����
A. 1 molҺ̬����������������ȫȼ������ˮ�������ų�642 kJ��������N2H4(l)+O2(g) ===N2(g)+2H2O(g) ��H��+642 kJ��mol��1
B. 12 gʯīת��ΪCOʱ���ų�110.5 kJ��������2C(ʯī��s)+O2(g) ===2CO(g) ��H����110.5 kJ��mol��1
C. ��֪��H2(g)+ ![]() O2(g) ===H2O(l) ��H����286 kJ��mol��1������2H2O(l) ===2H2(g)+O2(g)����H��+572 kJ��mol��1
O2(g) ===H2O(l) ��H����286 kJ��mol��1������2H2O(l) ===2H2(g)+O2(g)����H��+572 kJ��mol��1
D. ��֪N2(g)+3H2(g) ![]() 2NH3(g) ��H����92.4 kJ��mol��1������һ�����������ܱ������г���0.5 mol N2(g)��1.5 mol H2(g)��ַ�Ӧ�ų�46.2 kJ������
2NH3(g) ��H����92.4 kJ��mol��1������һ�����������ܱ������г���0.5 mol N2(g)��1.5 mol H2(g)��ַ�Ӧ�ų�46.2 kJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������SO2ͨ�벻ͬ��Һ�е�ʵ���������ý��۴�����ǣ� ��
ѡ�� | ��Һ | ���� | ���� |
A | H2O2��Һ | ���������� | SO2��H2O2����Ӧ |
B | H2S��Һ | ��������ɫ���� | SO2�������� |
C | ����KMO4��Һ��Һ | ��ɫ��ȥ | SO2�л�ԭ�� |
D | ���з�̪��NaOH��Һ | ��Һ��ɫ��ȥ | SO2����������������� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ͽ�1 mol AB(g)�����еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA��B���ļ��ܡ��±��г���һЩ��ѧ���ļ���E��
��ѧ�� | H��H | Cl��Cl | O===O | C��Cl | C��H | O��H | H��Cl |
E/kJ��mol��1 | 436 | 247 | x | 330 | 413 | 463 | 431 |
��ش��������⣺
��ͼ��ʾij��Ӧ�������仯��ϵ����˷�ӦΪ________(����ȡ����ȡ�)��Ӧ�����Ц�H��______________(�ú���a��b�Ĺ�ϵʽ��ʾ)��
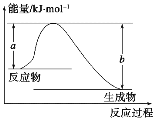
��ͼʾ�б�ʾ��Ӧ H2(g)��O2(g)===H2O(g) ��H����241.8 kJ��mol��1����b��________kJ��mol��1��x��__________��
(3)��ʷ�����á��ؿ���������������һ��������CuCl2����������450 �����ÿ����е��������Ȼ��ⷴӦ����������Ӧ�Ļ�ѧ����ʽΪ___________________________________���������¶Ⱥ�ѹǿ�Է�Ӧ�ȵ�Ӱ�죬���������е��й����ݣ����㵱��Ӧ����1 mol����ת��ʱ����Ӧ�������仯Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̼һ��Ҳ����һϵ���⻯����NH3��N2H4��N3H5��N4H6�ȡ�
��1��N3H5�ĵ���ʽΪ_______��
��2����֪4NH3(g)��5O2(g)===4NO(g)��6H2O(g)����H1��a kJ/mol��K1����
4NH3(g)��3O2(g)===2N2(g)��6H2O(g)����H2��b kJ/mol��K2����
д��N2��O2��Ӧ����1 mol NO������Ȼ�ѧ��ʽ��ʽΪ________________________��
��3����֪NH3��H2OΪһԪ���N2H4��H2OΪ��Ԫ�����ˮ��Һ�е�һ�����뷽��ʽ��ʾΪN2H4��H2O��H2O![]() N2H5��H2O����OH�������������������(N2H6Cl2)��Һ������Ũ���ɴ�С��˳��Ϊ___________________________________��
N2H5��H2O����OH�������������������(N2H6Cl2)��Һ������Ũ���ɴ�С��˳��Ϊ___________________________________��
��4����ͼ��ʾ���������̶��������������������ƶ���M��N�����������������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)
2NH3(g)
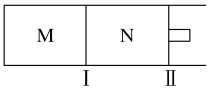
����M��N�У���ͨ��2 mol N2��6 mol H2����ʼM��N�ݻ���ͬ���������¶Ȳ��䡣��ƽ��ʱH2��ת������(H2)ΪM________N(��������������������)��
������ij�����£���ӦN2(g)��3H2(g)![]() 2NH3(g)������N�дﵽƽ�⣬��������к���1.0 mol N2��0.4 mol H2��0.2 mol NH3����ʱ�ݻ�Ϊ2.0 L����������µ�ƽ�ⳣ��Ϊ_______��
2NH3(g)������N�дﵽƽ�⣬��������к���1.0 mol N2��0.4 mol H2��0.2 mol NH3����ʱ�ݻ�Ϊ2.0 L����������µ�ƽ�ⳣ��Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���á���-Ferrozine����������ڼ�ȩ������ԭ�����£�

����˵��������ǣ� ��
A. ��״���£�11.2LCO2�к�̼��˫������ĿΪ6.02��1023
B. 30gHCHO������ʱת�Ƶ�����ĿΪ4��6.02��1023
C. ��Ӧ�ٵĻ�ѧ����ʽΪ2Ag2O+HCHO=4Ag+CO2+H2O
D. �����ϣ�����HCHO ������Fe3+�����ʵ���֮��Ϊ4��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ���ѻ���˼��������о������N4���ӣ���ṹΪ�������壨��ͼ��ʾ��������������ƣ���֪����1molN��N������193kJ����������1molN��N������941kJ������������˵������ȷ���ǣ� ��

A. N4��N2��Ϊͬ�������� B. 1molN4����ת��ΪN2ʱҪ�ų�724kJ����
C. N4���N2�ǻ�ѧ�仯 D. N4��N2��Ϊͬλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������½��з�Ӧ��COCl2(g) ![]() Cl2(g)��CO(g)����2.0 L�����ܱ������г���1.0 mol COCl2(g)����Ӧ�����в�õ��й����ݼ��±���
Cl2(g)��CO(g)����2.0 L�����ܱ������г���1.0 mol COCl2(g)����Ӧ�����в�õ��й����ݼ��±���
t/s | 0 | 2 | 4 | 6 | 8 |
n(Cl2)/mol | 0 | 0.30 | 0.39 | 0.40 | 0.40 |
����˵������ȷ����( )
A. ����Cl2��ƽ����Ӧ���ʣ�0��2s��2��4s��B. 0��2s COCl2��ƽ���ֽ�����Ϊ0.15mol��L��1��s��1
C. 6sʱ����Ӧ�ﵽ�����D. �������£�COCl2�����ת����Ϊ40%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������Ҵ�����������������ȼ�գ��õ�CO��CO2��ˮ��������Ϊ27.6g��������ˮ������Ϊ10.8g����CO��������( )
A. 1.4gB. 2.2gC. 4.4gD. ��2.2g��4.4g֮��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com