
����Ŀ����������(Na2FeO4)��һ�����͡���Ч��ˮ����������ˮ��Ӧ�Ļ�ѧ����ʽΪ4Na2FeO4��10H2O===4Fe(OH)3����3O2����8NaOH������Ʊ�Na2FeO4װ��ʾ��ͼ��ͼ��ʾ��
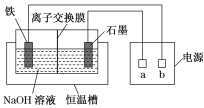
(1)a�ǵ�Դ��________(����������������)�������ʱ��ʯī�缫������Һ�ļ���________(������ǿ��������������������)��
(2)���缫�ķ�ӦʽΪ_________________________________________________��
(3)ά��һ���ĵ���ǿ�Ⱥ͵���¶ȣ�NaOH��ʼŨ�ȶ�Na2FeO4Ũ��Ӱ����ͼ(���Һ�����ͬ������½���ʵ��)��
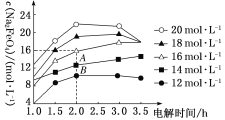
�ٵ��3.0 h�ڣ���NaOH��ʼŨ������Na2FeO4Ũ�ȱ仯������________(������������������������С��)��
�ڵ�NaOH��ʼŨ��Ϊ16 mol��L��1ʱ��1.0��2.0 h������Na2FeO4��������__________mol��L��1��h��1��
(4)�ᴿ�������Na2FeO4�������ؽᾧ�����ˡ�ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��________(����)��Һ���������
A��Fe(NO3)3�� ��B��NH4Cl�� ��C��CH3COONa
(5)��������������Ҳ�����Ƶ�Na2FeO4��
��֪��2H2(g)��O2(g)===2H2O(l)����H��a kJ��mol��1
NaCl(aq)��H2O(l)===NaClO(aq)��H2(g)����H��b kJ��mol��1
4Na2FeO4(aq)��10H2O(l)===4Fe(OH)3(s)��3O2(g)��8NaOH(aq)����H��c kJ��mol��1
��Ӧ2Fe(OH)3(s)��3NaClO(aq)��4NaOH(aq)===2Na2FeO4(aq)��3NaCl(aq)��5H2O(l)����H��_______kJ��mol��1��
���𰸡��� ��ǿ Fe��8OH����6e��===FeO![]() ��4H2O ���� 8 C ��
��4H2O ���� 8 C ��![]() a��3b��
a��3b��![]() c
c
��������
��ⷨ�Ʊ�Na2FeO4����Ԫ�ػ��ϼ����ߣ���ʧ���ӷ���������Ӧ���������缫��������ʯī�缫Ϊ������
(1) ��ⷨ�Ʊ�Na2FeO4����Ԫ�ػ��ϼ����ߣ��������缫��������ʯī�缫Ϊ������a�ǵ�Դ�ĸ��������ʱ��������Ӧʽ��![]() ��ʯī�缫������Һ�ļ�����ǿ��
��ʯī�缫������Һ�ļ�����ǿ��
(2)���缫��������������ʧ��������Na2FeO4��������ӦʽΪFe��8OH����6e��===FeO![]() ��4H2O��
��4H2O��
(3)�ٸ���ͼʾ�����3.0 h�ڣ���NaOH��ʼŨ������Na2FeO4Ũ������
�ڵ�NaOH��ʼŨ��Ϊ16 mol��L��1ʱ��1.0��2.0 h��Na2FeO4��Ũ�ȱ仯����16 mol��L��1��8 mol��L��1=8 mol��L��1��1.0��2.0 h������Na2FeO4��������8 mol��L��1��1h=8mol��L��1��h��1��
(4)��4Na2FeO4��10H2O===4Fe(OH)3����3O2����8NaOH��Ӧ��֪����������������FeO42-��ˮ��Ӧ�������ü�����Һϴ����ã�Fe(NO3)3��NH4Cl��Һ�����ԣ� CH3COONa��Һ�ʼ��ԣ���ѡC��
(5)��2H2(g)��O2(g)===2H2O(l)����H��a kJ��mol��1
��NaCl(aq)��H2O(l)===NaClO(aq)��H2(g)����H��b kJ��mol��1
��4Na2FeO4(aq)��10H2O(l)===4Fe(OH)3(s)��3O2(g)��8NaOH(aq)����H��c kJ��mol��1
���ݸ�˹���ɣ�-����2 -����3-����![]() ��2Fe(OH)3(s)��3NaClO(aq)��4NaOH(aq)===2Na2FeO4(aq)��3NaCl(aq)��5H2O(l)����H����
��2Fe(OH)3(s)��3NaClO(aq)��4NaOH(aq)===2Na2FeO4(aq)��3NaCl(aq)��5H2O(l)����H����![]() a��3b��
a��3b��![]() c kJ��mol��1��
c kJ��mol��1��


 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
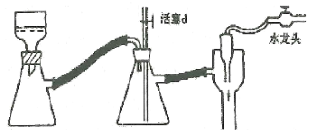
����Ŀ��ij����С�鹫˾������Li-SO2Cl2���õ�أ���ʾ��ͼ��ͼ��ʾ����֪��ط�ӦΪ��2Li+SO2Cl2��2LiCl+SO2�������������д������

A. ��ع���ʱ����������Li������������Ӧ
B. ���������Һ��ΪLiCl��ˮ��Һ��������ܻ����
C. ��ع���ʱ��������﮵缫�����ߡ����ء�̼��
D. ��ع��������У�ʯī�缫��ӦʽΪSO2Cl2+2e=2Cl+SO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
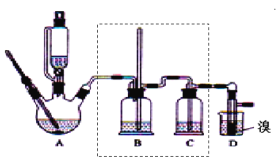
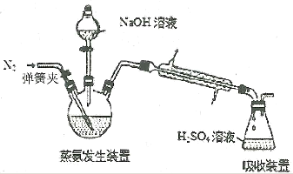
����Ŀ��ʵ�����Ʊ�1��2���������飬�����������Ҵ����Ʊ���ϩ��������ϩ�����������Ʊ�1��2���������飬װ����ͼ��ʾ���й������б������ʾ���ش��������⣺
�Ҵ� | 1��2���������� | ���� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
�ܶ�/gcm-3 | 0.79 | 2.2 | 0.71 |
�е�/�� | 78.5 | 132 | 34.6 |
�۵�/�� | -130 | 9 | -116 |
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����__��
a.������Ӧ b.�ӿ췴Ӧ�ٶ�
c.��ֹ�Ҵ��ӷ� d.���ٸ�������������
��2����װ��A�г���Ũ������Ҵ��⣬��Ӧ����__����Ŀ����__��װ��A�����ɸ��������ѵĻ�ѧ��Ӧ����ʽΪ__��
��3��ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2��Ϊ����֤SO2�Ĵ��ڲ���ȥSO2�Ժ�����Ӧ�ĸ��ţ�ijͬѧ��A��D֮�������B��C����װ�ã�����B��C�пɷֱ�ʢ��___��
a.����KMnO4��ˮ b.Ʒ���NaOH��Һ
c.����KMnO4��NaOH��Һ d.Ʒ�������KMnO4
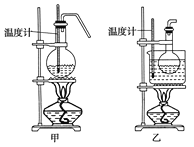
��4���ס�����װ�þ�������ʵ��������ˮ�Ҵ���ȡ��ϩ����ͼ���ø���ԡ����(���ͷе�290�棬�۵�18.17��)���������¶ȴﵽ��Ӧ�¶�ʱ����ʢ����ˮ�Ҵ���Ũ������Һ����ƿ��������У��ܿ�ﵽ��Ӧ�¶ȡ��ס�����װ����Ƚϣ���װ������Щ�ŵ�__��д����ʵ��������ˮ�Ҵ���ȡ��ϩ�Ļ�ѧ����ʽ___��
��5����1��2����������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ú���Ӧ��__�㣻�����������������������ѡ�����__�ķ�����ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������H2O2�����������ڴ��������Ȼ�林����£�ѡ�����̿��Ϊ�����Ʊ����Ȼ���������(III)�����(���ԵĶ����ܰ����������Ϊ���Ե������ܰ������)���������£�

��֪��Co(NH3)6Cl3�ڲ�ͬ�¶���ˮ�е��ܽ��������ͼ��

(һ)���Ȼ���������(III)�������Ʊ�
(1)�������Ҫ��ȴ��10���ٻ����ر߽������H2O2��Һ������������Ŀ���ǣ�________��
(2)ʵ�����Ʊ����Ȼ���������(III)�ܷ�Ӧ��ѧ����ʽΪ��_______________________��
(3)ʵ�����1Ϊ______________������2��[Co(NH3)6]Cl3��Һ�м���Ũ�����Ŀ����____________________________________________��
(4)ʵ�����õ���ѹ����װ����ͼ����������ϻ���;ֹͣ����ʱ��Ӧ����ȡ����ȷ����������_________________��
(��)��Ʒ��NH3�����IJⶨ
�ֳ�ȡ0.1000g��Ʒ����������ƿ�з������·�Ӧ��
[Co(NH3)x]Cl3+3NaOH=Co(OH)3��+xNH3��+3NaCl(����ͼ)����ƿ��װ��10.00mL c mol��L-1 H2SO4��������ƿ��ʹNH3��ȫ�ݳ����μ�2��ָʾ������0.5000mol��L-1 NaOH����Һ�ζ����ζ��ﵽ�յ�ʱ����NaOH��ҺV mL��
(5)���й���ʵ���˵������ȷ����______________��
A.�ڢٲ�����NH4Cl��Һ�м�����ϸ��CoCl2��6H2O���壬Ŀ���Ǽ��ٹ�����ܽ�
B.ԭ��NH4Cl����Ҫ����������NH3��H2O�ĵ��룬���������ɶ����ܰ��������γ�Cu(OH)2
C.��ѹ�����漰ת����Һ�������ǣ�����������ת����Һ����ˮ��ͷ������Һ������ʱ��ת�Ƴ���
D.�ڢܲ���ʹ�ú�������ķ�ˮ���ٽ���Ʒ������
E.�ζ�ʱ�����2��ָʾ���Ƿ�̪
(6)����ʵ��(��)�����ݼ��㣺������NH3����������Ϊ__________(�ú���ĸ��ʽ�ӱ�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������п�ͼ��ϵ��գ���֪��Ӧ![]() ��
��![]() ���ҹ���ҵ�����е���Ҫ��Ӧ��X������Ϊ��ɫ��ζ��Һ�壻C��ɫ��Ӧ����ʻ�ɫ��JΪ���ɫ������D��E������Ϊ���壬��E��ʹƷ����Һ��ɫ��A�����н�������Ԫ�أ����н���Ԫ�ص���������ԼΪ
���ҹ���ҵ�����е���Ҫ��Ӧ��X������Ϊ��ɫ��ζ��Һ�壻C��ɫ��Ӧ����ʻ�ɫ��JΪ���ɫ������D��E������Ϊ���壬��E��ʹƷ����Һ��ɫ��A�����н�������Ԫ�أ����н���Ԫ�ص���������ԼΪ![]() ��
��
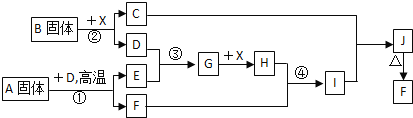
��1��![]() �Ļ�ѧʽΪ______��
�Ļ�ѧʽΪ______��
��2������A���������Ļ�ѧ�������B���ƣ���A�ĵ���ʽΪ______��
��3����Ӧ![]() �����ӷ���ʽ��______��
�����ӷ���ʽ��______��
��4����Ӧ![]() �����ӷ���ʽ��______��
�����ӷ���ʽ��______��
��5����֪ÿ����![]() ���ų�
���ų�![]() ��������Ӧ
��������Ӧ![]() ���Ȼ�ѧ����ʽΪ��______��
���Ȼ�ѧ����ʽΪ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����״���£���һʢ�е����NO��NO2���Թܵ�����ˮ���У���ַ�Ӧ��������������ȷ����(���Թ��е����ʲ����Թ�����ɢ)
A.�˷�Ӧ��ˮ�Ȳ����������ֲ��ǻ�ԭ��
B.�Թ�����Һ�����ʵ����ʵ���Ũ��Ϊ![]() mol/L
mol/L
C.��Һ���ռ�Թ��ݻ�������֮��
D.�����Թ��е����廻Ϊ�Ȼ��⣬��ˮ�����Թ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2A ![]() B��C��ijһ�¶�ʱ���ﵽƽ�⡣
B��C��ijһ�¶�ʱ���ﵽƽ�⡣
(1)���¶����ߣ�ƽ��������Ӧ�����ƶ���������Ӧ��________��Ӧ(��������������������)��
(2)��BΪ���壬��Сѹǿƽ�����淴Ӧ�����ƶ�����A��______̬��
(3)��A��B��C��Ϊ���壬���������ƽ��______�ƶ�(����������������������������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ӦN2O4(g)![]() 2NO2(g)����H��0��ƽ����ϵ��������(m��)�������ʵ���(n��)֮��M(M=
2NO2(g)����H��0��ƽ����ϵ��������(m��)�������ʵ���(n��)֮��M(M=![]() )��ͬ�¶�����ѹǿ�ı仯������ͼ��ʾ������˵����ȷ����:
)��ͬ�¶�����ѹǿ�ı仯������ͼ��ʾ������˵����ȷ����:
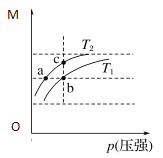
A. �¶ȣ�T1��T2
B. ƽ�ⳣ����K(a)��K(b)��K(c)
C. ��Ӧ���ʣ�vb��va
D. ��M=69 g��mol��1ʱ��n(NO2)��n(N2O4)��1��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ʻ����ʼ�S-�տ����Ƽ����ɱ�֤�ʻ�ʢ����S-�տ��صķ��ӽṹ����ͼ�����й��ڸ����ʵ�˵������ȷ���ǣ� ��
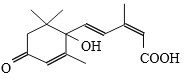
A.�����ʽΪC15H20O4
B.���ܷ����ӳɷ�Ӧ�����ܷ���ȡ����Ӧ
C.1mol��������ȫȼ�գ���Ҫ����403.2L����
D.����ʹ��ɫʯ���Լ��Ժ�ɫ������ʹ����KMnO4��Һ��ɫ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com