
����Ŀ��ʵ������H2O2�����������ڴ��������Ȼ�林����£�ѡ�����̿��Ϊ�����Ʊ����Ȼ���������(III)�����(���ԵĶ����ܰ����������Ϊ���Ե������ܰ������)���������£�

��֪��Co(NH3)6Cl3�ڲ�ͬ�¶���ˮ�е��ܽ��������ͼ��

(һ)���Ȼ���������(III)�������Ʊ�
(1)�������Ҫ��ȴ��10���ٻ����ر߽������H2O2��Һ������������Ŀ���ǣ�________��
(2)ʵ�����Ʊ����Ȼ���������(III)�ܷ�Ӧ��ѧ����ʽΪ��_______________________��
(3)ʵ�����1Ϊ______________������2��[Co(NH3)6]Cl3��Һ�м���Ũ�����Ŀ����____________________________________________��
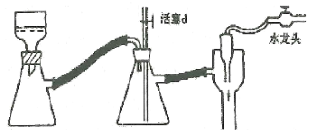
(4)ʵ�����õ���ѹ����װ����ͼ����������ϻ���;ֹͣ����ʱ��Ӧ����ȡ����ȷ����������_________________��
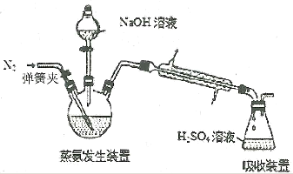
(��)��Ʒ��NH3�����IJⶨ
�ֳ�ȡ0.1000g��Ʒ����������ƿ�з������·�Ӧ��
[Co(NH3)x]Cl3+3NaOH=Co(OH)3��+xNH3��+3NaCl(����ͼ)����ƿ��װ��10.00mL c mol��L-1 H2SO4��������ƿ��ʹNH3��ȫ�ݳ����μ�2��ָʾ������0.5000mol��L-1 NaOH����Һ�ζ����ζ��ﵽ�յ�ʱ����NaOH��ҺV mL��
(5)���й���ʵ���˵������ȷ����______________��
A.�ڢٲ�����NH4Cl��Һ�м�����ϸ��CoCl2��6H2O���壬Ŀ���Ǽ��ٹ�����ܽ�
B.ԭ��NH4Cl����Ҫ����������NH3��H2O�ĵ��룬���������ɶ����ܰ��������γ�Cu(OH)2
C.��ѹ�����漰ת����Һ�������ǣ�����������ת����Һ����ˮ��ͷ������Һ������ʱ��ת�Ƴ���
D.�ڢܲ���ʹ�ú�������ķ�ˮ���ٽ���Ʒ������
E.�ζ�ʱ�����2��ָʾ���Ƿ�̪
(6)����ʵ��(��)�����ݼ��㣺������NH3����������Ϊ__________(�ú���ĸ��ʽ�ӱ�ʾ)��
���𰸡���ֹ�¶ȹ���H2O2�ֽ⣬NH3��H2O�ֽ⣻���ͷ�Ӧ���ʣ���ֹ��Ӧ���ھ��� 2CoCl2��6H2O+10NH3+2NH4Cl+H2O2 2[Co(NH3)6]Cl3+14H2O ���ȹ��� ������[Co(NH3)6]Cl3�����������ͬ����ЧӦ������߲��� �ȴ���d����ر�ˮ��ͷ DE (0.34c-0.085V) ��100%
2[Co(NH3)6]Cl3+14H2O ���ȹ��� ������[Co(NH3)6]Cl3�����������ͬ����ЧӦ������߲��� �ȴ���d����ر�ˮ��ͷ DE (0.34c-0.085V) ��100%
��������
��1����Ӧ����H2O2����ˮ�����������ʶ����Ȳ��ȶ���ʹ��ʱ��Ҫע�ⷴӦ�����⣻���⣬����������Ϊ�˷�ֹ��Ӧ���ھ��ң�
��2���������е���Ϣ���÷�Ӧ�ķ�Ӧ����CoCl2��6H2O��NH3��NH4Cl��H2O2����������[Co(NH3)6]Cl3��H2O2�Ļ�ԭ����һ����H2O���ݴ�д����ѧ����ʽ����ƽ���ɣ�
��3����[Co(NH3)6]Cl3�ͻ���̿�Ļ����Һ�м��뺬ŨHCl��ˮ��������I�õ�[Co(NH3)6]Cl3��Һ�������IΪ���ȹ��ˣ���[Co(NH3)6]Cl3��Һ�м���ŨHCl�����Դٽ�[Co(NH3)6]Cl3�����������ͬ����ЧӦ����
��4����������ϻ���;ֹͣ����ʱ��Ӧ����ȡ����ȷ�����������ȴ���d����ر�ˮ��ͷ��
��5��A��������������뵽��Һ�У�Ŀ���Ǽӿ��ܽ⣻
B����ˮ�д���:NH3+H2O![]() NH3��H2O
NH3��H2O![]() NH4++OH-������ƽ���ƶ���ԭ��������
NH4++OH-������ƽ���ƶ���ԭ��������
C����ʵ�������ȷ��
D�����뺬HCl�ķ�ˮ�������˵õ�[Co(NH3)6]Cl3��Һ����Ȼ�ò�������Ϊ���������壻
E���ζ��ﵽ�յ�ʱ����Һ����NH4+�������ԣ�����ʹ�ü�����ָʾ������
��6�����ݵζ����㼴�ɡ�
��1����Ӧ����H2O2����ˮ�����������ʶ����Ȳ��ȶ���ʹ��ʱ����������Ҫ���£���ֹ���������ʷֽ⣻���⣬����������Ϊ�˷�ֹ��Ӧ���ھ��ң�
��2���÷�Ӧ�Ļ�ѧ����ʽΪ��2CoCl2��6H2O+10NH3+2NH4Cl+H2O2 2[Co(NH3)6]Cl3+14H2O��
2[Co(NH3)6]Cl3+14H2O��
��3����[Co(NH3)6]Cl3�ͻ���̿�Ļ����Һ�м��뺬ŨHCl��ˮ��������I�õ�[Co(NH3)6]Cl3��Һ�������IΪ���ȹ��ˣ���[Co(NH3)6]Cl3��Һ�м���ŨHCl�����Դٽ�[Co(NH3)6]Cl3�������������߲��ʣ�
��4����������ϻ���;ֹͣ����ʱ��Ӧ����ȡ����ȷ�����������ȴ���d����ر�ˮ��ͷ��
��5��A��ȷ��
B����ˮ�д���:NH3+H2O![]() NH3��H2O
NH3��H2O![]() NH4++OH-������Һ�м���NH4Cl����������NH3��H2O�ĵ��룬��СOH-��Ũ�ȣ�����NH3��Ũ�ȣ��Դٽ������ܰ����������ɣ�����Cu(OH)2�����ɣ�B��ȷ��
NH4++OH-������Һ�м���NH4Cl����������NH3��H2O�ĵ��룬��СOH-��Ũ�ȣ�����NH3��Ũ�ȣ��Դٽ������ܰ����������ɣ�����Cu(OH)2�����ɣ�B��ȷ��
C��ȷ��
D�����뺬HCl�ķ�ˮ�������˵õ�[Co(NH3)6]Cl3��Һ����ò��費��Ϊ�˴ٽ���Ʒ��������D����
E���ζ��ﵽ�յ�ʱ����Һ����NH4+�������ԣ�����ʹ�ü�����ָʾ�����������÷�̪��E����
��ѡDE��
��6��n(NaOH)=V��10-3L��0.5mol/L=0.5V��10-3mol������2NaOH-H2SO4����ʣ��H2SO4 0.25V��10-3mol��������NH3��H2SO4��(0.01c-0.25V��10-3)mol������2NH3-H2SO4�������յ�NH3��(0.02c-0.5V��10-3)mol![]() ��100%=(0.34c-0.085V) ��100%��
��100%=(0.34c-0.085V) ��100%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ��ɱ���ܱ������г���2mol��������4mol����������T1�ͺ�ѹP1�����½������·�Ӧ��![]() ��H1= -208.8kJ�� mol-1����ƽ��ʱ���������Ϊ2L������ת����Ϊ50%�������й�˵����ȷ����( )
��H1= -208.8kJ�� mol-1����ƽ��ʱ���������Ϊ2L������ת����Ϊ50%�������й�˵����ȷ����( )
A.������������������ٱ仯ʱ��˵���÷�Ӧ����ƽ��״̬
B.��Ӧ��ϵ��������Ũ�ȱ��ֲ��䣬˵���÷�Ӧ����ƽ��״̬
C.��ԭƽ������ϣ����������г���3mol��������ƽ�������ƶ�
D.��ԭƽ������ϣ������¶ȣ�����ת��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ṥҵβ���е�NO��NO2����������γɹ⻯ѧ�������ƻ�������ȡ���������������Һ�Ժ����������β�����д�������Ӧ�Ļ�ѧ����ʽ���£�NO2+NO+2NaOH=2NaNO2+H2O��2NO2+2NaOH=NaNO2+NaNO3+H2O������Ҳ����������������������磬������һ�������ɷ������·�Ӧ��4NH3+6NO=5N2+6H2O����һ����NO��NO2�Ļ������ͨ��300mL5mol/LNaOH��Һ�У�ǡ�ñ���ȫ���ա�����˵������ȷ����
A.���ð���������������ʱ�������������ԭ��Ӧ
B.������Һ��NaNO3��NaNO2�����ʵ���֮�ȿ���Ϊ2��1
C.���ð������������谱���ڱ�״���µ��������Ϊ39.2L
D.ԭ���������NO�ڱ�״���µ��������Ϊ16.8L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���������Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ���������ͼ��ʾ��

(1)ָ����ȡ��Ĺ������й�ʵ����������ƣ���__________����__________��
(2)�������пɹ�ѡ����л��Լ���_________(�����)��
A.�ױ����ƾ� B.���Ȼ�̼���� C.���͡����� D.���͡�����
(3)������ʹ������еĵ�����ת��Ϊ�л���Һ�еĵⵥ�ʵ�ʵ�飬ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг�������ҩƷ����ȱ�ٵIJ���������__________��___________��
(4)Ҫ�ӵ�ı���Һ����ȡ��ͻ��ձ�������Ҫ������������������������ʱ����ʹ��ˮԡ���ȣ�Ŀ����__________�����̬����________�оۼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������β������Ҫ�ɷ���CO��SO2����������ȣ���ѧ����-ֱ��������Ⱦ�����Ч������
(1)���ð�ˮ���Խ�SO2�������������գ�ԭ������ͼ��ʾ��
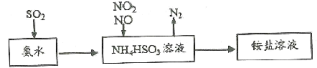
��д��NO2��NO�������1��1������ʱ��Ӧ�����ӷ���ʽ_________________________��
(2)���й�����Ŀǰ���ڳ����Զ�������(TiO2)���ֽ�����β�����о���
�ټ�֪��2NO(g)+O2(g)=2NO2(g) ��H1=-113.0kJ�� mol-1
2SO2(g)+O2(g)=2SO3(l) ��H2=-288.4kJ�� mol-1
N2(g)+O2(g)![]() 2NO(g) ��H3=+180.5kJ�� mol-1
2NO(g) ��H3=+180.5kJ�� mol-1
���жϷ�ӦNO2(g)+SO2(g)=NO(g)+SO3(l) ��H4���ڵ������ܷ��Է�����_______(��ܡ���)��������__________________________��
�ڼ�֪TiO2��β������ԭ��Ϊ��
2CO(g)+O2(g)![]() 2CO2(g)��2H2O(g)+4NO(g)+3O2(g)
2CO2(g)��2H2O(g)+4NO(g)+3O2(g)![]() 4HNO3(g)
4HNO3(g)
i����һ�������£�ģ��CO��NO�Ľ��⣬�õ�������(��ת����)��ʱ��仯��ͼ��ʾ��
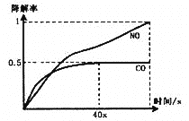
��Ӧ40�������������N2Ũ��������HNO3����Ũ���������ͣ����û�ѧ����ʽ����ϻ�ѧ��Ӧԭ��֪ʶ���Ϳ��ܵ�ԭ��____________________________________________��
ii�����������Ҳ�ɽ���CO����ͼΪ�ڲ�ͬ������϶�����������(��������)�ڲ�ͬ�¶��£���Ӧ��ͬʱ�䣬���CO�����ʱ仯�����ͼ���ش��������⣺
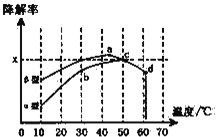
��֪��50��ʱ��������������������У�ƽ��ʱO2Ũ��Ϊ0.01mol��L-1������¶���CO���ⷴӦ��ƽ�ⳣ��____________________(�ú�x�Ĵ���ʽ��ʾ)���������������������Ϊ���壬��TiO2��Ϊ��Ч�����õ�TiO2���ܣ���10��60�淶Χ�ڽ���ʵ�飬����ͼ�����߶�����Ӱ��������ʾ��������CO���������¶ȱ仯�����߿��ܳ��ֵ��������Χ(ʾ����![]() )_____________________��
)_____________________��
(3)������ͼ��ʾװ��(�缫��Ϊ���Ե缫)Ҳ������SO2�����������ų�����Һ����NO2��b���ĵ缫��ӦʽΪ_______________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼװ���У���ƿ�г�����������a������ͷ�ι��е�Һ��b������ƿ�ڣ���������ƿ��Ȼ����ɼ�f���ձ��е�Һ��b����Ȫ״��������ռ���������ƿ��a��b�ֱ������
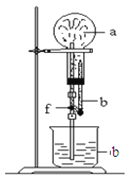
��������a | Һ��b | |
A | NO2 | ˮ |
B | C12 | ����ʳ��ˮ |
C | NH3 | ˮ |
D | CO2 | 4 mol��L��1NaOH��Һ |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(Na2FeO4)��һ�����͡���Ч��ˮ����������ˮ��Ӧ�Ļ�ѧ����ʽΪ4Na2FeO4��10H2O===4Fe(OH)3����3O2����8NaOH������Ʊ�Na2FeO4װ��ʾ��ͼ��ͼ��ʾ��
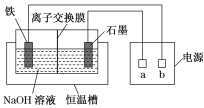
(1)a�ǵ�Դ��________(����������������)�������ʱ��ʯī�缫������Һ�ļ���________(������ǿ��������������������)��
(2)���缫�ķ�ӦʽΪ_________________________________________________��
(3)ά��һ���ĵ���ǿ�Ⱥ͵���¶ȣ�NaOH��ʼŨ�ȶ�Na2FeO4Ũ��Ӱ����ͼ(���Һ�����ͬ������½���ʵ��)��
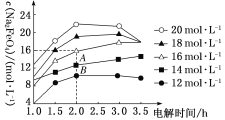
�ٵ��3.0 h�ڣ���NaOH��ʼŨ������Na2FeO4Ũ�ȱ仯������________(������������������������С��)��
�ڵ�NaOH��ʼŨ��Ϊ16 mol��L��1ʱ��1.0��2.0 h������Na2FeO4��������__________mol��L��1��h��1��
(4)�ᴿ�������Na2FeO4�������ؽᾧ�����ˡ�ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��________(����)��Һ���������
A��Fe(NO3)3�� ��B��NH4Cl�� ��C��CH3COONa
(5)��������������Ҳ�����Ƶ�Na2FeO4��
��֪��2H2(g)��O2(g)===2H2O(l)����H��a kJ��mol��1
NaCl(aq)��H2O(l)===NaClO(aq)��H2(g)����H��b kJ��mol��1
4Na2FeO4(aq)��10H2O(l)===4Fe(OH)3(s)��3O2(g)��8NaOH(aq)����H��c kJ��mol��1
��Ӧ2Fe(OH)3(s)��3NaClO(aq)��4NaOH(aq)===2Na2FeO4(aq)��3NaCl(aq)��5H2O(l)����H��_______kJ��mol��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
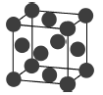
����Ŀ�����־���ľ����ṹ��ͼ��ʾ�������й�˵����ȷ���ǣ� ��
A.ͼ����ʾ����Ļ�ѧʽΪA3B4C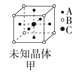
B.ͼ����ʾ�������������Ӹ�����Ϊ1��1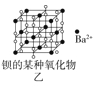
C.ͼ����ʾCaF2������Ca2+��λ��Ϊ4��F-��λ��Ϊ8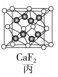
D.ͼ����ʾ�������ڼ������ѻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�[Cu(NH3) 4]( NO3) 2��Һ����ϲ�����Cu���Ƶ�һ�ֺ���ɫ���塣
��1����̬Cu2����������Ų�ʽ��______��
��2������ɫ����ľ�����ͼ��ʾ��д���þ���Ļ�ѧʽ��______��
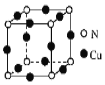
��3��[Cu(NH3)4]2+����λ��Ŀռ�ṹ�ṹΪ______
��4��![]() ��Nԭ�ӹ�����ӻ�������______����
��Nԭ�ӹ�����ӻ�������______����![]() ��Ϊ�ȵ������һ�ַ���Ϊ______���ѧʽ����
��Ϊ�ȵ������һ�ַ���Ϊ______���ѧʽ����
��5������������ˮ��ԭ����______��
��6������ͭ�Ķѻ���ʽ��ͼ��ʾ�����þ�������a��658pm����þ����ܶ�Ϊ________(�г��������ʽ)g��cm��3��
 ��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com