
����Ŀ�����仯������������;����ش��������⡣
![]() ��̬�����Ӽ۲���ӵĹ������ʽΪ____���������������ܼ��Ĺ����Ϊ____��
��̬�����Ӽ۲���ӵĹ������ʽΪ____���������������ܼ��Ĺ����Ϊ____��
![]() ������������������
������������������![]() ��
��![]() ��
��![]() ��
��![]() �ȣ��������ʵ��۵��ɸߵ��͵�˳������Ϊ__________��
�ȣ��������ʵ��۵��ɸߵ��͵�˳������Ϊ__________��
![]() ��Ǧ��
��Ǧ��![]() ����Ǧ
����Ǧ![]() ��һ�ֱȽϳ����Ŀ�����ܷ�ӦΪ��
��һ�ֱȽϳ����Ŀ�����ܷ�ӦΪ��![]() Ũ
Ũ![]() ����
����![]() ����λԭ����__________����һ������
����λԭ����__________����һ������![]() __________
__________![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ��
��![]() ������ӻ���ʽΪ______________��������̬���ӵĿռ乹����
������ӻ���ʽΪ______________��������̬���ӵĿռ乹����![]() ��ͬ����__________��
��ͬ����__________��
A.SnCl2 B.SO3 ![]()
![]()
![]() ��Ǧ�������������ͼ��ʾ�������Ӳ�ȡ���������ѻ���Ǧ�����������������γɵ�__________��϶�С���֪�����ܶ�Ϊ��g��cm��3�������ӵ�������ֵΪ
��Ǧ�������������ͼ��ʾ�������Ӳ�ȡ���������ѻ���Ǧ�����������������γɵ�__________��϶�С���֪�����ܶ�Ϊ��g��cm��3�������ӵ�������ֵΪ![]() ��������������Ǧ��������ľ���Ϊ__________nm��
��������������Ǧ��������ľ���Ϊ__________nm��

���𰸡�![]() 3
3 ![]()
![]()
![]()
![]() AC ������
AC ������ 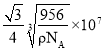
��������
�Ż�̬��ԭ�ӵ����Ų�ʽ1s22s22p63s23p4�����ӵ����Ų�ʽ1s22s22p63s23p6�����Ӽ۲�����Ų�ʽΪ3s23p6�����Ӽ۵��ӵĹ������ʽΪ![]() ��
��
�ʴ�Ϊ![]()
�������������ܼ�Ϊ3p�ܼ���3p�ܼ��Ĺ����Ϊ3�������
�ʴ�Ϊ3��
�����ʵ��۵�Ƚϵ�һ��ԭ��Ϊ���Ӿ����۵���ڷ��Ӿ����۵㣬���ƺ������߶�Ϊ���Ӿ��壬�������Ӱ뾶�;����ܱȽϣ������Ӱ뾶С�ڼ����Ӱ뾶�����ƾ����ܴ����ؾ����ܣ���������۵�������۵㣬���������S8���Ƿ��Ӿ��壬��ɽṹ���Ƶķ��Ӿ��壬��Է�������Խ���۵�Խ�ߣ����S8�۵���ڶ��������۵㣬���������۵�ߵ�˳��ΪNa2S > K2S > S8 > SO2��
�ʴ�ΪNa2S > K2S > S8 > SO2
����H2[PbCl4]����λԭ����Cl���ʴ�ΪCl��
ͬ���ڵ�һ�����ܴ�����������IIA>III A,V A> VI A���ȵĵ�һ�����ܴ�����ĵ�һ�����ܣ��ʴ�Ϊ![]() ��
��
H2S�е��Ӷ���
![]()
����H2S���ӻ���ʽΪSP3��
�ʴ�ΪSP3��
H2S�е��Ӷ���=2��2=4��VSEPRģ��Ϊ�������壬��ȥ���Թ¶Ե��ӣ�Ϊ��V���νṹ��SnCl2�е�����=2��2=4��ͬ��Ϊ��V���νṹ��SO3�е�����=3��0=3����Ϊƽ�������νṹ��O3����Ϊ2�������γ���![]() ��������ԭ��
��������ԭ��![]() �γ�ʱ�����������ӣ���˻�ʣ���������Ӽ�һ�Թ¶Ե��ӣ����Ӷ���=2��1=3����Ϊ��V���νṹ��CH4�е�����=4��0=4����Ϊ��������ṹ��
�γ�ʱ�����������ӣ���˻�ʣ���������Ӽ�һ�Թ¶Ե��ӣ����Ӷ���=2��1=3����Ϊ��V���νṹ��CH4�е�����=4��0=4����Ϊ��������ṹ��
�ʴ�ΪAC
�ȸ��ݷ�Ǧ������������������Ӳ�ȡ���������ѻ����ĸ������Ӻ�Ǧ�����γ���������ṹ��Ǧ�������������϶�У��ʴ�Ϊ�����壻
��֪�����ܶ�Ϊ��g��cm��3��
������������Ǧ��������ľ���Ϊ��Խ��ߵ��ķ�֮һ���þ�������4��Ǧ���Ӻ�4�������ӣ����ⳤΪa��
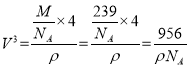
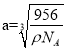
![]()
����������������Ǧ��������ľ���Ϊ
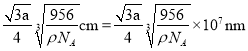
�ʴ�Ϊ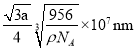



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ģ�ij�о�С������ͼװ�ý���SO2��FeCl3��Һ��Ӧ�����ʵ��(�г�װ������ȥ)��
(1)ͨ������SO2ʱC�й۲쵽��������______________________��
(2)������������С��ͬѧ��ΪSO2��FeCl3��Һ����������ԭ��Ӧ��
��д��SO2��FeCl3��Һ��Ӧ�����ӷ���ʽ��_______________________��
�������ʵ�鷽��������Fe2�����ɣ�_________________________��
����С��ͬѧ��C�Թܷ�Ӧ�����Һ�м��������ữ��BaCl2��Һ�������ְ�ɫ����������֤����Ӧ������SO42����������_____(����������������������)��������______��
(3)Dװ���е���©����������__________________________��
(4)Ϊ����֤SO2���л�ԭ�ԣ�ʵ���п��Դ���FeCl3���Լ���________(����ĸ)��
a��ŨH2SO4 b������KMnO4��Һ c����ˮ d��NaCl��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��A����Է�������Ϊ140������̼����������Ϊ0.857��A������������̼ԭ�Ӳ�����ֱ��������A��һ������������ֻ����G��G��ʹʯ����Һ��졣

�����
(1)A�ķ���ʽ___________________��
(2)������A��G�Ľṹ��ʽ�� A___________________��G___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
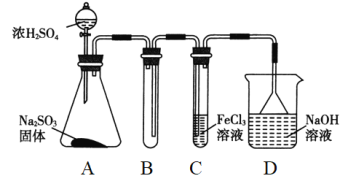
����Ŀ���縡ѡ���۷�����������ˮ�Ĺ���ԭ����ͼ��ʾ������˵������ȷ��( )
A. ���缫�ĵ缫��ӦʽΪFe��2e����Fe2��
B. ͨ������ʯī�ĵ缫��ӦʽΪ��CH4+4CO32����8e�� ==5CO2+2H2O
C. Ϊ����ǿ����ˮ�ĵ���������������ˮ�м�������ʳ��
D. �����ʯī�缫����44.8L���壬������0.5mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
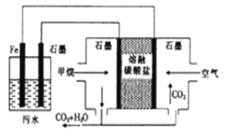
����Ŀ����ͼ��һ���绯ѧ���̵�ʾ��ͼ����ش��������⣺

(1)ͼ�м׳���OH-����_________��������CH3OH������O2������
(2)д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ��__________________________________
(3)A�缫��������_____ �� �ҳ��з�Ӧ�Ļ�ѧ����ʽΪ_____________����Ҫʹ��Һ�ָ����ǰ��״̬��Ӧ���ҳ��м���________(д��ѧʽ)��
(4)���ҳ���B(Ag)����������10.8g���׳�������������O2�����Ϊ________L(��״��)����ʱ������ij�缫����ij������������е�ij����Һ������____________
A. MgSO4 B.CuSO4 C.NaCl D. AgNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��M��WΪ���ֶ�����Ԫ�ء�Xԭ�ӵ�����������Ӳ�����ͬ��Wԭ�Ӻ����������Mԭ��������������2����Y��Z��M��W�����ڱ��е����λ����ͼ��ʾ������˵������ȷ����

A. ԭ�Ӱ뾶��W>Y>Z>M>X
B. ���ȶ��ԣ�XM>X2Z���е㣺X2Z>YX3
C. X��Y��Z����Ԫ���γɵĻ������в����ܺ����Ӽ�
D. YM3��WM4������ÿ��ԭ������������8���ӽṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ĵ��ʼ�һЩ�������ڹ�ũҵ��������������ҪӦ�ã��ش���������
(1)�ǰ� ( 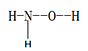 ) �Dz��ȶ��İ�ɫ��Ƭ״����״�ᾧ��������������������ˮ��
) �Dz��ȶ��İ�ɫ��Ƭ״����״�ᾧ��������������������ˮ��
���ǰ������в���sp3�ӻ���ԭ����____________________���ǰ���������ˮ����Ҫԭ����________________________________��
�����ǰ�����ɵ�Ԫ���У�����ͬһ����Ԫ�صĵ��ĵ�����(I4)�ϴ����______________(��Ԫ�ط��ű�ʾ)��
(2) ���![]() ���ڷ��������Ǻ������л��l mol����к���
���ڷ��������Ǻ������л��l mol����к���![]() �������ʵ���Ϊ_________ mol��
�������ʵ���Ϊ_________ mol��
(3)��֪��ÿ1mol�������ʷֽ�Ϊ��̬��̬ԭ�����������ֱ�Ϊ
NO2 | CO | CO2 | NO |
812kJ | 1076kJ | 1490kJ | 632kJ |
��NO2 + CO ![]() CO2 + NO
CO2 + NO
��N2(g)+O2(g) ![]() 2NO(g) ��H��+179.5 kJ/mol
2NO(g) ��H��+179.5 kJ/mol
��2NO(g) +O2(g)![]() 2NO2(g) ��H��-112.3 kJ/mol
2NO2(g) ��H��-112.3 kJ/mol
��д��NO��CO��Ӧ��������Ⱦ��������Ȼ�ѧ����ʽ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ�д��ڵ���ƽ��CH3COOH![]() H����CH3COO��������ȷ���ǣ� ��
H����CH3COO��������ȷ���ǣ� ��
A. ������Һ������Ũ�ȵĹ�ϵ���㣺c��H������c��OH������c��CH3COO����
B. ������0.10mol/L��CH3COOH��Һ�м�ˮϡ�ͣ���Һ��c��OH������С
C. CH3COOH��Һ�м�������CH3COONa���壬ƽ�������ƶ�
D. ������pH��2��CH3COOH��Һ��pH��12��NaOH��Һ�������Ϻ���Һ��pH��7
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com