
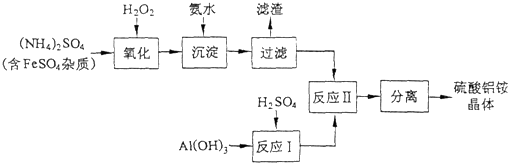
| 4.53g |
| 906g/mol |
| 2.16g-2.07g |
| 18g/mol |


 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Һ����ʱ����Һ©�����²�Һ����¶˷ų����ϲ�Һ����Ͽڵ��� |
| B��ij�����м���ϡ���ᣬ������ɫ��ζ����ʹ����ʯ��ˮ����ǵ����壬֤���ù�����һ������CO32- |
| C���������Һ���ȼ���BaCl2��Һ��������ɫ�������ټ����������ᣬ�������ܽ⣬֤����Һ��һ������SO42- |
| D����Ũ��������һ�����ʵ���Ũ�ȵ�ϡ����ʱ����Ũ����ϡ�ͺ�Ӧ��ȴ�����º���ת�Ƶ�����ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH3��CH2��3CH2OH |
| B��CH3CH2CH��CH3��CH2OH |
| C����CH3��2CHCH2CH2OH |
| D����CH3CH2��2CHOH |
�鿴�𰸺ͽ���>>
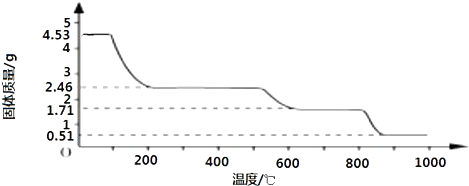
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣�����ͲӦ����
��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣�����ͲӦ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
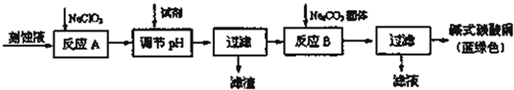
| ���� | Cu��OH��2 | Fe��OH��2 | Fe��OH��3 |
| ��ʼ����pH | 6.0 | 7.5 | 1��4 |
| ������ȫpH | 13 | 14 | 3.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
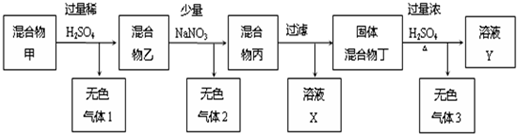
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
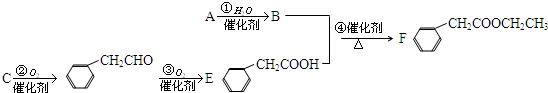
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
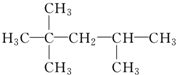 ��������
���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
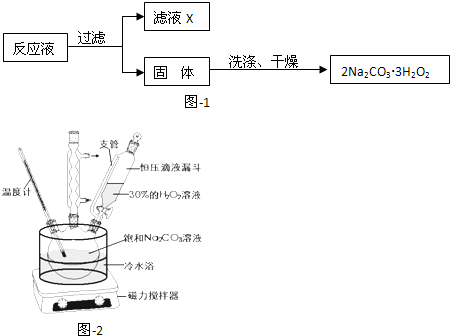
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ�� | �� �� �� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com