
����Ŀ����һ���¶��£���a.���� b������ c�����������
(1)������c(H��)��ͬʱ�����ʵ���Ũ���ɴ�С��˳����____________��
(2)ͬ�����ͬ���ʵ���Ũ�ȵ������ᣬ�к�NaOH�������ɴ�С��˳����______________��
(3)�����������ʵ���Ũ����ͬʱ��c(H��)�ɴ�С��˳����__________��
(4)������c(H��)��ͬ�����Ҳ��ͬʱ���ֱ����������п����ͬ״���²������������ɴ�С��˳����__________________��
(5)��c(H��)��ͬ�����������ˮϡ����ԭ����100����c(H��)�ɴ�С��˳����____________��
(6)��c(H��)��ͬ�������ͬʱ��ͬʱ������״���ܶȡ�������ȫ��ͬ��п����������ͬ�����H2(��ͬ״��)����ʼʱ��Ӧ���ʵĴ�С��ϵΪ_______����Ӧ����ʱ��ij��̹�ϵ��________��
���𰸡� c>a>b b>a��c b>a>c c>a��b c>a��b a��b��c a��b>c
����������1��������c(H��)��ͬʱ�����ڴ����ȫ���룬����c(CH3COOH)Ҫ��c(HCl)��c(H2SO4)������������Ϊ��Ԫ�ᣬ����c(HCl)>c(H2SO4)��������Ũ���ɴ�С��˳����c(CH3COOH) > c(HCl)>c(H2SO4)����ȷ�𰸣�c>a>b ��
��2��ͬ�����ͬ���ʵ���Ũ�ȵ������ᣬ���ᡢ���ᡢ�������ʵ�����ȣ����������������ӵ��������ᡢ����������������2�������������ʵ�����������ʵ�����ȣ������к�NaOH�������ɴ�С��˳��ΪH2SO4>HCl=CH3COOH����ȷ�𰸣�b>a��c��
(3)�����������ʵ���Ũ����ͬʱ�����ݵ��뷽��ʽ��֪��HCl==H++Cl-��H2SO4=2H++SO42- ��CH3COOH![]() CH3COO-+H+��c(H��)�ɴ�С��˳����H2SO4> HCl> CH3COOH����ȷ����b>a>c��
CH3COO-+H+��c(H��)�ɴ�С��˳����H2SO4> HCl> CH3COOH����ȷ����b>a>c��
(4)���ᡢ����Ϊǿ�ᡢ����Ϊ���ᣬ����c(H��)��ͬ�����Ҳ��ͬʱ�����������࣬���ᡢ�����ṩ�������ӵ�����ȣ������������зֱ����������п����ͬ״���²������������ɴ�С��˳���Ǵ���>����=��������ȷ����c>a��b��
(5)��c(H��)��ͬ�����������ˮϡ����ԭ����100����ǿ����c(H��)���͵�ԭ����1/100,���Ǵ���Ϊ���ᣬ���ڵ���ƽ�⣬��ˮϡ��ƽ�����ƣ�c(H��)���ٲ���ԭ����1/100,��ˣ�c(H��)�ɴ�С��˳���Ǵ���>����=�����ȷ�𰸣�c>a��b��
(6)п�������ӷ�Ӧ��Ӧ������������c(H��)��ͬ�������ͬʱ��ͬʱ������״���ܶȡ�������ȫ��ͬ��п����������ͬ�����H2(��ͬ״��)����ʼʱ��Ӧ������ȣ����ڴ����Ũ�ȴ������������Ũ�ȣ����Դ�����п��Ӧ���ʽϴ��õ�ʱ��̣������������п��Ӧ������ȣ��õ�ʱ�䳤����ȷ�𰸣�a��b��c��a��b>c��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�����ר�Һ�°�ġ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�������NaHCO3��NaCl��NH4C1�������ܽ�ȵIJ��죬��ʳ�Ρ�������������̼��Ϊԭ���Ƶ�NaHCO3�������������������A��B�� C�� D�ĸ�װ�ÿ���װ��ʵ����ģ�⡰�����Ƽ����ȡNaHCO3��ʵ��װ�á�װ���зֱ�ʢ�������Լ���B��ϡ���C�����ᡢ̼��ƣ�D�������ı���ʳ��ˮ��ˮ��

�������ڲ�ͬ�¶��µ��ܽ��(g/100 gˮ)��(˵������>35 �� NH4HCO3���зֽ�)

��ش��������⣺
(1)װ�õ�����˳��Ӧ��__________(����ĸ)��
(2)Aװ����ʢ�ŵ��Լ���__________����������___________________��
(3)��ʵ������У���Ҫ����D�¶���30�桫35�棬ԭ����_________________��
(4)��Ӧ��������ƿ������ˮ�У�����NaHCO3���塣������ˮϴ��NaHCO3�����Ŀ���dz�ȥ____(�����Ի�ѧʽ��ʾ)��
(5)����ƿ�еIJ�����˺����õ�ĸҺ�к���___________(�Ի�ѧʽ��ʾ)�������Ȼ��⣬������_________������ʹNaCl��Һѭ��ʹ�ã�ͬʱ�ɻ���NH4Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȷ��ʾ���з�Ӧ�����ӷ���ʽ��
A. �������ƹ�����ˮ��Ӧ��2O22-+2H2O=4OH-+O2��
B. ������[KAl(SO4)2]��Һ����μ���Ba(OH)2��Һ��SO42-ǡ�ó�����ȫ��2Al3++3SO42-+3Ba2++6OH- =2Al(OH)3��+3BaSO4��
C. ��ͭ���缫���CuSO4��Һ��2Cu2++2H2O![]() 2Cu+O2��+4H+
2Cu+O2��+4H+
D. FeSO4������Һ��¶�ڿ����У�4Fe2++O2+4H+=4Fe3++2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����º������ò����������Ƶ������������д�����ϸ��,�����á�ӫ���ء���ӫ����ø����ⷨ�����г������⺬ϸ�����ٽ��м��:�ٽ�������ĥ�����Ĵ���,ȡһ��������Һ����ֹ��ȼ�(�ⶨ����ǿ�ȵ�����)��Ӧ����,����������ӫ���غ�ӫ����ø,�����������½��з�Ӧ;�ڼ�¼����ǿ�Ȳ�����ATP����;�۲����ϸ���������������ش���������:
(1)ӫ���ؽ���____�ṩ��������ͱ�����,��ӫ����ø���������γ�����ӫ���ز��ҷ���ӫ�⡣���ݷ���ǿ�ȿ��Լ����������֯��ATP�ĺ���,ԭ���Ƿ���ǿ����ATP������____________(����/����);����ATP�������������ϸ��������������:ÿ��ϸ��ϸ����ATP����__________��
(2)��ӫ���ء���ӫ����ø����ⷨ�����漰������ת����_________;����ϸ����ATP��ˮ��һ����________(���ܷ�Ӧ����ܷ�Ӧ)����ϵ��
(3)�о���Ա�ò�ͬ��������ӫ����ø��,�ⶨøŨ���뷢��ǿ����ͼ��ʾ��
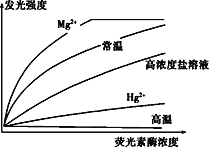
���и�Ũ������Һ��ϡ�ͺ�ø���Կ��Իָ�,���º�Hg2+������ø���Բ��ɻָ�����Ҫ��ʡӫ����ø������,����ʹ��____����;Hg2+������ø���Խ��Ϳ�������Ϊ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪As2O3��Zn���Է������·�Ӧ��As2O3+6Zn+6H2SO4![]() 2AsH3��+6ZnSO4+3H2O��
2AsH3��+6ZnSO4+3H2O��
(1)����˫���ŷ��������ת�Ƶķ������Ŀ____________________________________��
(2)As2O3��������Ӧ����ʾ������������____________(�����)��
A�������� B����ԭ�� C������ D������
(3)�÷�Ӧ������������__________����ԭ������________��
(4)������0.2 mol AsH3����ת�Ƶĵ�����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH4��+6HCHO=3H��+6H2O+(CH2)6N4H�� ��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᣬij��ȤС���ü�ȩ������������ʵ�飺
����I ��ȡ��Ʒ1.500g��
����II ����Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
����� ��ȡ25.00mL��Ʒ��Һ��250mL��ƿ�У�����10mL20�������Լ�ȩ��Һ��ҡ�ȡ�����5 min��,����1~2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
(1)���ݲ������գ�
�ٵ���ζ��յ�ʱ�����Ӽ�ʽ�ζ��ܶ�����������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�ڴﵽ�ζ��յ�ʱ�����ּ�ʽ�ζ��ܵļ�������ݣ���ζ�ʱ��ȥNaOH����Һ�����__________ (�ƫ����ƫС������Ӱ�족)
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�___________
�ܵζ��ﵽ�յ��жϣ�__________________________________________
(2)�ζ�������±���ʾ��
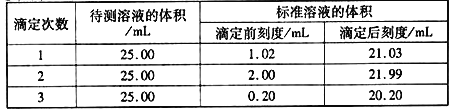
��NaOH����Һ��Ũ��Ϊ0.1010mol��L��1�����Ʒ�е�����������Ϊ______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������NH4I�����ܱ������У���һ���¶��·������з�Ӧ����NH4I(s)![]() NH3(g)+HI(g) ��2HI(g)
NH3(g)+HI(g) ��2HI(g)![]() H2(g)+I2(g) �ﵽƽ��ʱ��c(H2)��0.5 mol��L-1��c(HI)��4 mol��L-1��������¶��·�Ӧ����ƽ�ⳣ��Ϊ
H2(g)+I2(g) �ﵽƽ��ʱ��c(H2)��0.5 mol��L-1��c(HI)��4 mol��L-1��������¶��·�Ӧ����ƽ�ⳣ��Ϊ
A. 9 B. 16 C. 20 D. 25
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��0.1000mol��L-1��H3PO4��Һ��0.1000mol��L-1��NaOH��Һ���еζ�����ζ���������ͼ��ʾ[��֪���ζ�����a=n(�ζ�Һ������)/n(���ζ�����)]������˵����ȷ����

A. n=1ʱ��c(Na+)��c(H2PO4-)��c(H3PO4)��c(HPO42-)
B. n=1.5ʱ��2c(Na+)��3c(H3PO4)+3c(HPO42-)
C. n=2ʱ��c(Na+)��c(HPO42-)��c(PO43-)��c(H2PO4-)
D. n=3ʱ��c(OH-)=c(H+)+c(HPO42-)+c(H2PO4-)+c(H3PO4)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������˵������ȷ���� �� ��
A. Ǧ���طŵ�ʱ�����Ĺ���ԭ��Ϊ��PbO2+4H++SO42-+2e-�TPbSO4+2H2O������ʱ�����ķ�Ӧʽ��PbSO4+2e-�TPb+SO42-
B. ����п�̸ɵ�طŵ�ʱ��������Ӧʽ��Zn-2e-�TZn2+
C. ���������طŵ�ʱ������Ӧʽ��H2-2e-+2OH-�T2H2O
D. ij����ӵ�ص��ܷ�ӦʽΪ��Li+2Li0.35NiO2![]() 2Li0.85NiO2��������ʱ������ӦʽΪ��2Li0.85NiO2-e-�T2Li0.35NiO2+Li+
2Li0.85NiO2��������ʱ������ӦʽΪ��2Li0.85NiO2-e-�T2Li0.35NiO2+Li+
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com