
����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ����û�ѧ����ش��������⣺
�� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
��1�� �ڢۡ���Ԫ���У�ԭ�Ӱ뾶������__________����Ԫ�ط��ţ���
��2�� ��Ԫ�ص�����������Ӧ��ˮ���������⻯����������M��M�к��еĻ�ѧ��������________________________��
��3��д��Ԫ�آٺ͢�ĵ����ڼ��������·�Ӧ���ɵĻ�����ĵ���ʽ��_____________��
��4�� �ۡ��ݡ��ߡ����γɵ����ӣ���뾶��С�����˳����________�������ӷ��ţ�
��5�� �١�����Ԫ������������Ӧ��ˮ������������ǿ����_____________�������ʻ�ѧʽ���������Ե�����������_________�������ʻ�ѧʽ�����û�������NaOH��Һ��Ӧ�����ӷ���ʽΪ___________��
��6�� �õ���ʽ��ʾԪ�آ�����γɻ�����Ĺ���_____________________________��
��7��д����ҵұ���ݵĻ�ѧ����ʽ��_______________________________________��
���𰸡�Ca ���Ӽ������ۼ� ![]() Al3+ �� Mg2+ �� O2�� �� N3�� HClO4 Al(OH)3 Al(OH) 3+OH��=AlO2��+2H2O
Al3+ �� Mg2+ �� O2�� �� N3�� HClO4 Al(OH)3 Al(OH) 3+OH��=AlO2��+2H2O ![]() 2Al2O3(����)
2Al2O3(����)  4Al+3O2��
4Al+3O2��
��������
��Ԫ�����ڱ��Ľṹ��֪����ΪNaԪ�ء���ΪKԪ�ء���ΪMgԪ�ء���ΪCaԪ�ء���ΪAlԪ�ء���ΪCԪ�ء���ΪNԪ�ء���ΪOԪ�ء���ΪClԪ�ء���ΪArԪ�أ����Ԫ�����ڱ��Ľṹ�������⡣
��ΪNaԪ�ء���ΪKԪ�ء���ΪMgԪ�ء���ΪCaԪ�ء���ΪAlԪ�ء���ΪCԪ�ء���ΪNԪ�ء���ΪOԪ�ء���ΪClԪ�ء���ΪArԪ�أ�
(1)��֪��ΪMgԪ�ء���ΪCaԪ�ء���ΪAlԪ�ء���ΪCԪ�ء���ΪNԪ�أ����ڢۡ���Ԫ���У�CaΪ������������Ԫ�أ���ԭ�Ӱ뾶��Mg����ԭ�Ӱ뾶������Ca��
(2)��ΪNԪ�أ�NԪ�ص�����������Ӧ��ˮ���������⻯����������ΪNH4NO3�����еĻ�ѧ�����������Ӽ������ۼ���
(3)��ΪNaԪ�ء���ΪOԪ�أ�Na��O2�ڼ��������·�Ӧ���ɵĻ�����ΪNa2O2���������ͻ���������ʽΪ![]() ��
��
(4)��ΪMgԪ�ء���ΪAlԪ�ء���ΪNԪ�ء���ΪOԪ�أ�Mg2+��Al3+��N3-��O2-�Ľṹ��ͬ���˵���������Ӱ뾶С����뾶��С�����˳����Al3+ �� Mg2+ �� O2�� �� N3����
(5)Ԫ�صķǽ�����Խǿ��������������Ӧ��ˮ����������Խǿ����OԪ��������̬�����ڢ١����г�O�⣬ClԪ�صķǽ�������ǿ����Ԫ������������Ӧ��ˮ������������ǿ����HClO4��Al�����Խ�����������Ե�����������Al(OH)3��Al(OH)3��NaOH��Һ��Ӧ����ƫ�����ƺ�ˮ��������Ӧ�����ӷ���ʽΪAl(OH) 3+OH��=AlO2��+2H2O��
(6)��ΪMgԪ�ء���ΪClԪ�أ���Ԫ���γɵĻ�����ΪMgCl2������ʽ�γɻ��������Ϊ![]() ��
��
(7)��ΪAlԪ�أ���ҵ���õ���������ķ���ұ��Al����Ӧ�Ļ�ѧ����ʽΪ2Al2O3(����)  4Al+3O2����
4Al+3O2����


 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£���a L�ܱ������м���1 mol X�����2 mol Y���壬�������·�Ӧ��X(g)+2Y(g)![]() 2Z(g)������˵���˷�Ӧ�ﵽƽ��ı�־�ǣ� ��
2Z(g)������˵���˷�Ӧ�ﵽƽ��ı�־�ǣ� ��
A.������ѹǿ����ʱ��仯
B.�����ڸ����ʵİٷֺ�������ʱ��仯
C.������X��Y��Z��Ũ��֮��Ϊ1��2��2
D.v��(X)=2v��(Y)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ط�[KCr(SO4)2�B12H2O]�������֯�ȹ�ҵ���й㷺����;��ijѧϰС���û�ԭK2Cr2O7,������Һ�ķ����Ʊ����ط���װ����ͼ��ʾ��
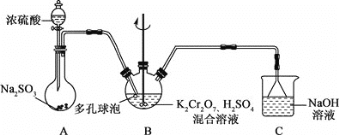
�ش��������⣺
(1)A��ʢװŨ���������������________��
(2)װ��B�з�Ӧ�����ӷ���ʽΪ_________________________________��
(3)��Ӧ������Bװ���л�ȡ���ط�����ķ����ǣ��������м����Ҵ����������壬���ˡ�ϴ�ӡ����¸���õ���Ʒ��
�ټ����Ҵ����ٽ��й��˵�Ŀ����____________��
�ڵ��¸����ԭ����_________________________��
(4)���ط���Ʒ�и������IJⶨ��ȡmg��Ʒ������ƿ��������ˮ�ܽ⣬����������Na2O2����������Ȼ���������ữʹ��Ԫ��ȫ�����Cr2O72-����c mol/L��FeSO4��Һ�ζ����յ㣬����FeSO4��Һv mL������Ʒ�и�����������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ���ǣ� ��
A.��0.1molL-1�İ�ˮ�м�����������粒��壬��Һ��![]() ����
����
B.����Сʵ�飺�������ǽ�����ʳ���У������ݲ�����˵��CH3COOH���������
C.�мס������ִ�����Һ����ü�pH=a���ҵ�pH=a+1���������к͵����ʵ���Ũ�ȵ������NaOH��Һ�������ļס�����������V(��)>10V(��)
D.�����ͬ��Ũ�Ⱦ�Ϊ0.1molL-1��NaOH��Һ����ˮ���ֱ�ϡ��m��n����ʹ��Һ��pH����Ϊ9����m<n
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ʵ�����CO��H2������������º����ܱ������н��з�Ӧ��CO��g��+2H2��g��![]() CH3OH��g����������ʵ��˵���˷�Ӧ�Ѵﵽƽ��״̬���ǣ� ��
CH3OH��g����������ʵ��˵���˷�Ӧ�Ѵﵽƽ��״̬���ǣ� ��
A.�����������ܶȱ��ֲ���
B.��CO��H2��CH3OH��ʾ��Ӧ������֮��Ϊ1��2��1
C.��λʱ��������nmolCO��ͬʱ����2nmolH2
D.��������ƽ����Է�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£�7.84L������ȫȼ�պ����ɶ�����̼��Һ̬ˮ��ͬʱ�ų�311.4kJ�������������ȼ�յ��Ȼ�ѧ����ʽ��( )
A. CH4+2O2��CO2+2H2O+311.4kJ
B. CH4(g)+2O2(g)��CO2(g)+2H2O(l)+311.4kJ
C. CH4(g)+2O2(g)��CO2(g)+2H2O(l)+889.7kJ
D. CH4(g)+2O2(g)��CO2(g)+2H2O(g)+889.7kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£���amolA��15molB�Ļ������ͨ��һ�̶����Ϊ5L���ܱ������У��������·�Ӧ��A��g��+3B��g��![]() 2C��g��
2C��g��
��1������Ӧ���е�10minʱ�ﵽƽ�⣬n1��A��=13mol��n1��C��=6mol��������a��ֵ___�����ʱ���ڵ�Cƽ����Ӧ����Ϊ___��
��2����Ӧ��ƽ��ʱ��B��ת����Ϊ�ֱ�Ϊ___��A���������Ϊ___��
��3�����в�����ʹ�÷�Ӧ�ķ�Ӧ�����������___��
A.���ܱ�������ͨ�뺤������ѹǿ
B.����A��Ũ��
C.�ʵ������¶�
D.�����������������10L
��4����Ӧ������������������ܶ���___������������������С����������������ͬ����ƽ����Է���������___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ���ı�
���ı�![]() ��Һ��
��Һ��![]() ����Һ��
����Һ��![]() Ũ�ȵĶ���ֵ
Ũ�ȵĶ���ֵ![]() ����Һ
����Һ![]() �ı仯��ϵ��ͼ��ʾ����
�ı仯��ϵ��ͼ��ʾ����![]() �����������������ǣ� ��
�����������������ǣ� ��
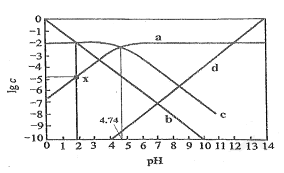
A. ![]() ʱ��
ʱ��![]()
B. ![]() ���볣����������Ϊ
���볣����������Ϊ![]()
C. ͼ�е�x��������ֵΪ![]()
D. ![]() ��
��![]() Լ������c����d���㴦�ĺ�����ֵ
Լ������c����d���㴦�ĺ�����ֵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��LiFePO4����Ϊ���������Ҳ�ص��������ϡ���������(��Ҫ�ɷ�ΪFeTiO3��Fe2O3������CuO��SiO2����)Ϊ��Ҫԭ������TiOSO4��ͬʱ�õ����̷�(FeSO4��7H2O)�������LiOH��Ӧ���Ƹ� LiFePO4�� LiFePO4���Ʊ���������ͼ��ʾ��

��ش��������⣺
(1)����ʱ FeTiO3�����ᷴӦ�Ļ�ѧ����ʽ�ɱ�ʾΪ____________________��
(2)�ټ���м��ԭ��Ŀ����__________���ڹ���ǰ��Ҫ���黹ԭ�Ƿ���ȫ����ʵ�����������Ϊ_________��
(3)�١���Ӧ����Ҫ����һ����˳�����FeSO4��Һ�������LiOH�������˳��ӦΪ____________________������������______________________________��
(4)�����е�ͭ�ᴿ���������ȡCu2O��Cu2O��һ�ְ뵼����ϣ�������ɫ��ѧ������Ƶ���ȡCu2O�ĵ���ʾ��ͼ���£�����ܷ�Ӧ��2Cu+H2O![]() Cu2O+H2�������װ����ͭ�缫Ӧ����ֱ����Դ��__________����ʯī�缫�ĵ缫��ӦʽΪ____________________������0. 1mol Cu2O����ʱ��·��ת��__________mol���ӡ�
Cu2O+H2�������װ����ͭ�缫Ӧ����ֱ����Դ��__________����ʯī�缫�ĵ缫��ӦʽΪ____________________������0. 1mol Cu2O����ʱ��·��ת��__________mol���ӡ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com