
����Ŀ����Ԫ���ܹ��γɶ��ֻ������ش��������⣺
![]() ����
����![]() ������ΪҺ̬���ڿ�����Ѹ����ȫȼ������
������ΪҺ̬���ڿ�����Ѹ����ȫȼ������![]() ��ͬʱ�ų������ȣ���������������ɴ��������ȼ�ϡ�
��ͬʱ�ų������ȣ���������������ɴ��������ȼ�ϡ�
��֪��![]() ��ȼ����Ϊ
��ȼ����Ϊ![]()
![]() ��
��![]()
![]() ��
��![]()
��![]() �ڿ�����ȼ��������̬ˮ���Ȼ�ѧ����ʽΪ______��
�ڿ�����ȼ��������̬ˮ���Ȼ�ѧ����ʽΪ______��
![]() ��ҵ�����ð�������������
��ҵ�����ð�������������![]() �ķ�ӦΪ
�ķ�ӦΪ![]()
![]() ��
��
![]() һ���¶��£���2L���������г���1mol
һ���¶��£���2L���������г���1mol![]() ��2mol
��2mol![]() ����������Ӧ��8min�ﵽƽ��ʱ�����
����������Ӧ��8min�ﵽƽ��ʱ�����![]() ��ת����Ϊ
��ת����Ϊ![]() ��
��![]() �ڣ���
�ڣ���![]() ��ʾ�ĸ÷�Ӧ����v
��ʾ�ĸ÷�Ӧ����v![]() ______��
______��
�����¶Ⱥ��ݻ����䣬����ƽ���������г���![]()
![]() ��
��![]() HCN����ʱ
HCN����ʱ![]() ______
______![]() ѡ����
ѡ����![]() ����
����![]() ������
������![]() ��
��![]() ��
��
![]() ��
��![]() �£���a
�£���a![]() ��NaCN��Һ��
��NaCN��Һ��![]() ������������ϣ���Ӧ������Һ
������������ϣ���Ӧ������Һ![]() ����a______
����a______![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ���ú�a�Ĵ���ʽ��ʾHCN�ĵ��볣��
���ú�a�Ĵ���ʽ��ʾHCN�ĵ��볣��![]() ______��
______��
![]() �ܹ���
�ܹ���![]() �γ�
�γ�![]() ��
��
![]() ��Һ�д���
��Һ�д���![]()
![]()
![]()
![]()
![]() ʱ����ƽ�ⳣ���ı���ʽΪ
ʱ����ƽ�ⳣ���ı���ʽΪ![]() ______��
______��
![]() ���������������кܴ��ԣ��绯ѧ����
���������������кܴ��ԣ��绯ѧ����![]() ��ԭ����ͼ��
��ԭ����ͼ��
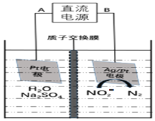
![]() ������ӦʽΪ______��
������ӦʽΪ______��
![]() ����������ת����3mol���ӣ���Ĥ������Һ�������仯��
����������ת����3mol���ӣ���Ĥ������Һ�������仯��![]() Ϊ______g��
Ϊ______g��
���𰸡�![]()
![]()
![]()
![]()
![]()
![]()
![]() 2NO2-+6e-+8H+=N2
2NO2-+6e-+8H+=N2![]() +4H2O 16
+4H2O 16
��������
![]() д��������ȼ���ȵ��Ȼ�ѧ����ʽ��Ȼ����ݸ�˹���ɼ��ɽ��
д��������ȼ���ȵ��Ȼ�ѧ����ʽ��Ȼ����ݸ�˹���ɼ��ɽ��
![]() ���ݷ�Ӧ����
���ݷ�Ӧ����![]() ���ɼ���������Ĺ�ϵ���ҳ����ĵ������������ٸ��ݹ�ʽ����¶Ȳ��䣬ƽ�ⳣ���Ͳ��䣬Ȼ���������Ũ�Ȼ���ƽ�ⳣ���Ĵ�С�Ƚϣ����ж�ƽ�����ƶ����Ӷ��ó�ʱ
���ɼ���������Ĺ�ϵ���ҳ����ĵ������������ٸ��ݹ�ʽ����¶Ȳ��䣬ƽ�ⳣ���Ͳ��䣬Ȼ���������Ũ�Ȼ���ƽ�ⳣ���Ĵ�С�Ƚϣ����ж�ƽ�����ƶ����Ӷ��ó�ʱ![]() ��
��![]() �Ĺ�ϵ����Һ�����������Һ�������������������ӵ���Դ�С���ٶ�
�Ĺ�ϵ����Һ�����������Һ�������������������ӵ���Դ�С���ٶ�![]() �������ǡ����ȫ��Ӧ���õ�NaCl��HCN�Ļ����Һ����ʱ��Һ�����ԣ������
�������ǡ����ȫ��Ӧ���õ�NaCl��HCN�Ļ����Һ����ʱ��Һ�����ԣ������![]() ʱ����
ʱ����![]() ���õ���غ�������غ����ҳ���Һ�е����Ӵ�С��ϵ��Ȼ���ڽ����볣������������ɣ�
���õ���غ�������غ����ҳ���Һ�е����Ӵ�С��ϵ��Ȼ���ڽ����볣������������ɣ�
![]() ƽ�ⳣ������������ƽ��Ũ�ȵ���֮�����Է�Ӧ��Ũ�ȵ���֮����Ȼ���ҳ�k��Ksp�Ĺ�ϵʽ������ã�
ƽ�ⳣ������������ƽ��Ũ�ȵ���֮�����Է�Ӧ��Ũ�ȵ���֮����Ȼ���ҳ�k��Ksp�Ĺ�ϵʽ������ã�
![]() ���Ĺ���ԭ����NO2-�������õ����ӣ�д���缫����ʽ��2NO2-+6e-+8H+=N2
���Ĺ���ԭ����NO2-�������õ����ӣ�д���缫����ʽ��2NO2-+6e-+8H+=N2![]() +4H2O���������������ʵı仯���ҳ����ʵ�ȥ������������IJ��
+4H2O���������������ʵı仯���ҳ����ʵ�ȥ������������IJ��
![]() �������֪��������ȼ����Ϊ
�������֪��������ȼ����Ϊ![]() ����
����![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
���ݸ�˹���ɣ�![]() ��
��![]() ��
��
�ʴ�Ϊ��![]() ��
��
![]() �ﵽƽ��ʱ�����ļ�������ʵ���
�ﵽƽ��ʱ�����ļ�������ʵ���![]() ���ɷ�Ӧ����ʽ��֪������
���ɷ�Ӧ����ʽ��֪������![]() �����ʵ���Ϊ�����3������
�����ʵ���Ϊ�����3������![]() ��0��8min�ȣ���������ʾ�ĸ÷�Ӧ����
��0��8min�ȣ���������ʾ�ĸ÷�Ӧ����![]() =
=![]() ,���ݷ�Ӧ����ʽ��������������֪���ﵽƽ��ʱ��
,���ݷ�Ӧ����ʽ��������������֪���ﵽƽ��ʱ��![]() ��
��![]() ��
��![]()
![]() ��
��![]() ����ƽ�ⳣ��
����ƽ�ⳣ��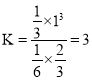 ������ƽ���������г�
������ƽ���������г�![]()
![]() ��
��![]() HCN��
HCN��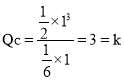 ��ƽ�ⲻ�ƶ���
��ƽ�ⲻ�ƶ���![]() ������������ȣ��ʴ�Ϊ��=��
������������ȣ��ʴ�Ϊ��=��
![]() ����
����![]() �������ǡ����ȫ��Ӧ���õ�NaCl��HCN�Ļ����Һ����ʱ��Һ�����ԣ����
�������ǡ����ȫ��Ӧ���õ�NaCl��HCN�Ļ����Һ����ʱ��Һ�����ԣ����![]() ʱ��
ʱ��![]() �������ϣ�����Ũ�Ⱦ����롣��Ӧ����Һ
�������ϣ�����Ũ�Ⱦ����롣��Ӧ����Һ![]() ��
��![]() �����ݵ���غ�ã�
�����ݵ���غ�ã�![]() ����
����![]() ��
��![]() �����������غ�ã�
�����������غ�ã�![]() ����
����![]() ��HCN�ĵ��볣��
��HCN�ĵ��볣��![]()
�ʴ�Ϊ��![]()
![]() ��
��
![]() ƽ�ⳣ������������ƽ��Ũ�ȵ���֮�����Է�Ӧ��Ũ�ȵ���֮������ƽ�ⳣ���ı���ʽ
ƽ�ⳣ������������ƽ��Ũ�ȵ���֮�����Է�Ӧ��Ũ�ȵ���֮������ƽ�ⳣ���ı���ʽ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ��װ��ͼ����Ϣ��֪�缫BΪ�����������õ����ӣ����缫��ӦʽΪ2NO2-+6e-+8H+=N2
��װ��ͼ����Ϣ��֪�缫BΪ�����������õ����ӣ����缫��ӦʽΪ2NO2-+6e-+8H+=N2![]() +4H2O���ʴ�Ϊ��2NO2-+6e-+8H+=N2
+4H2O���ʴ�Ϊ��2NO2-+6e-+8H+=N2![]() +4H2O��
+4H2O��
����ͼʾ��֪������Ϊ![]() ������3mol����ͨ��ʱ����������
������3mol����ͨ��ʱ����������![]() ��������3mol�����ӽ��������ң���������������27g��������
��������3mol�����ӽ��������ң���������������27g��������![]() ��֪������
��֪������![]() ��N2����14g��ͬʱ��
��N2����14g��ͬʱ��![]() ��3g���룬�������Ҽ�������Ϊ11g����Ĥ������Һ�������仯��
��3g���룬�������Ҽ�������Ϊ11g����Ĥ������Һ�������仯��![]() ���ʴ�Ϊ��16��
���ʴ�Ϊ��16��


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
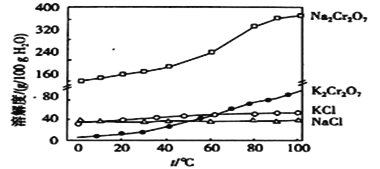
����Ŀ�������£����и���������ָ����Һ��һ���ܴ����������( )
��![]() ����Һ��
����Һ��![]()
��![]() ����Һ�У�
����Һ�У�![]()
��ˮ�����![]() Ũ��
Ũ��![]() ����Һ�У�
����Һ�У�![]()
�ܼ���![]() �ܷų�
�ܷų�![]() ����Һ�У�
����Һ�У�![]()
��ʹʯ�������Һ�У�![]()
��������Һ�У�![]()
A.�ڢ�B.ֻ�Т�C.�ڢܢ�D.�٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ظ����(![]() )���Ƹ﹤ҵ��ӡˢ��ҵ����ƹ�ҵ������Ҫ��;�ù�ҵ���Ը�����
)���Ƹ﹤ҵ��ӡˢ��ҵ����ƹ�ҵ������Ҫ��;�ù�ҵ���Ը�����![]() ����
����![]() ����)Ϊԭ���Ʊ��ظ���صĹ������£�
����)Ϊԭ���Ʊ��ظ���صĹ������£�
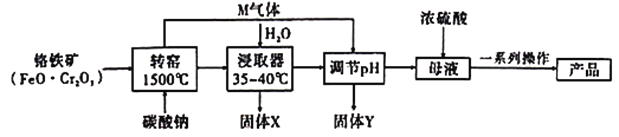
��֪����![]() �����й����ʵ��ܽ��������ͼ��
�����й����ʵ��ܽ��������ͼ��
��1��תҤ�з�������Ҫ��Ӧ���������������Ӧ�⣬����������Ӧ��
��![]() ��
��
��___![]() ___
___![]() +____
+____![]() ___
___![]() ___
___![]() ___________��
___________��
����ƽ��Ӧ�ڵĻ�ѧ����ʽ��
����д��������Ҫ��Ӧ�Ļ�ѧ����ʽ��_________________________________��
��2����������ѧ��ѧ�г��õIJ�������������ʵ�����н��������̼���ƹ����������գ����и�ʵ�������в���Ҫ����___________(�����)��
A.�մ����� B.������ C.���ż� D.������
��3������Y�ɷֵĻ�ѧʽΪ_________________��
��4����ĸҺ�м���Ũ���ᣬ�Ѹ�����ת��Ϊ�ظ����ƣ��Դ�ƽ��ĽǶ�˵��_______________________________________________________________________��
��5������һϵ�в������У�������һ���Ǽ���![]() ����øò�Ʒ��ԭ����____________________________��
����øò�Ʒ��ԭ����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʳ�û������к������ᣬ������һ�ֲ�����֬���ᣬ�����彡�����棬����ӽṹ��ͼ��ʾ������˵������ȷ���ǣ� ��
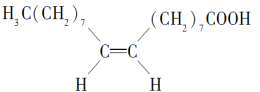
A.����ķ���ʽΪC18H34O2
B.�����������������Һ�����кͷ�Ӧ
C.1 mol�������2 mol H2�����ӳɷ�Ӧ
D.1 mol���Ϳ���3 mol���ᷢ��������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й���ͼ��ʾ��˵����ȷ����

A. ͼ���п�������N��ʱͭп�Ͻ�ʴ����������
B. ͼ���н�ͨ����ʱ��п��ʴ����������п�Ϸų��������������
C. ͼ���н�ͨK2ʱ����������ʴ��������������������
D. ͼ���н�ͨK1ʱ��ʯī����Χ��Һ��pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܱ�ʾH2(g)+ I2(g)![]() 2HI(g)�Ѿ��ﵽƽ��״̬�ı�־�ǣ� ��
2HI(g)�Ѿ��ﵽƽ��״̬�ı�־�ǣ� ��
��c(H2)=c(I2)=c(HI)ʱ
��c(H2)��c(I2)��c(HI)=1��1��2ʱ
��c(H2)��c(I2)��c(HI)������ʱ����ı�
�ܵ�λʱ��������nmolH2��ͬʱ����2nmolHI
�ݵ�λʱ��������nmolH2��ͬʱ����nmolI2
��Ӧ��v(H2)=v(I2)=1/2v(HI)
��һ��H-H�����ѵ�ͬʱ������H-I������
���¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯
���¶Ⱥ����һ��ʱ������������ɫ���ٱ仯
���¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯
����һ������������ƽ����Է����������ٱ仯
A.�ۢܢߢ��B.�ۢܢߢ�C.�ڢۢܢߢ�D.�ڢۢܢޢߢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������뵰�����йص���Ŀ��
��1����������ʱ�����ŵ��г�������ζ�����壬����������Ҫ����___________________���ѧʽ����˵���������к���___________________����Ԫ�����ƣ�Ԫ�ء�
��2����ʳ�ؽ����λ��ж���������Ϊ___________________��
��3��Ũ���ὦ��Ƥ���ϣ�ʹƤ������___________________ɫ����������Ũ����͵����ʷ�����___________________��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����ģ���������Ƽ��ԭ������NaCl��NH3��CO2��Ϊԭ�����Ƶ�NaHCO3����������������йط�Ӧ�Ļ�ѧ����ʽΪNH3��CO2��H2O=NH4HCO3��NH4HCO3��NaCl=NaHCO3����NH4Cl��2NaHCO3![]() Na2CO3��CO2����H2O��
Na2CO3��CO2����H2O��
��1������������Ӧԭ���������ͼ��ʾװ�ã���ȡ̼�����ƾ��壬C�ձ���ʢ�б�ˮ��D��װ��պϡ�������֬�ޣ�ͼ�мг�װ������ȥ��
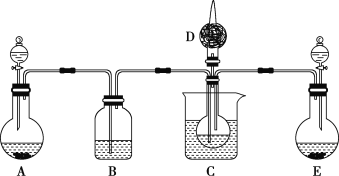
��E���Ʊ�����ʱ������ҩƷ��___(����ĸ)��
a��ʯ��ʯ b����ʯ�� c��ϡ���� d��Ũ��ˮ
��B��Ӧʢ��___��Һ����ʵ������У�Ӧ��C����ͨ��������__��
��Eװ����C��ͨ���ĵ��ܲ��ܲ���Һ���µ�ԭ����___��
��2����С��ͬѧΪ�˲ⶨC������̼�����ƾ���Ĵ���(���辧���в���̼��������)���������ָ����������Ϊ4g���ٽ�������ȵ��������ٱ仯ʱ���������÷�ĩ����Ϊmg��Ȼ�������ͼ��ʾʵ�飺
![]()
![]()
![]()
![]()
![]()
![]()
���ڲ������У�Ϊ���жϼ����Ȼ�����Һ�Ƿ��������ȷ��ʵ������ǣ��ڼ����Ȼ�����Һ��___��
�ڲ������漰�IJ�������Ϊ___��___�����
�����þ�����̼�����ƵĴ���Ϊ____%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯�������������й㷺Ӧ�á�
��1��Fe2+��̬�ĵ����Ų�ʽΪ___��
��2��ʵ������Fe3+���鱽�ӡ����ӷ�����̼ԭ�ӵ��ӻ���ʽΪ___��
��3����Fe��BNΪԭ�Ϻϳɵ������������ڹ�������������й㷺Ӧ�á�
��������(NH3BH3)Ϊԭ�Ͽ��Ի��BN��������ĽṹʽΪ___(��λ������������ʾ)��������������ˮ������Ҫԭ����___��
��������Ͱ���Ϊԭ�Ͽɺϳɰ����顣NH3����___���ӣ����������������Ǽ���������
����ͼΪFe��N���γɵ�һ�ֻ�����Ļ����ṹ��Ԫ���û�����Ļ�ѧʽΪ___��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com