
،¾جâؤ؟،؟ثل¼îضذ؛حµخ¶¨تاضذر§»¯ر§³£¼ûتµرé،£
¢ٌ.دآح¼±يت¾50mLثلت½µخ¶¨¹ـضذز؛أوµؤخ»ضأ£¬بç¹ûز؛أو´¦µؤ¶ءتتاa£¬شٍµخ¶¨¹ـضذت£سàز؛جهµؤجه»تا______mL،£
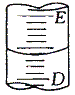
A،¢a B،¢´َسعa C،¢ذ،سع(50-a) D،¢´َسع(50-a)
¢ٍ.ؤ³ح¬ر§سû²â¶¨ؤ³إ¨ءٍثلرùئ·µؤخïضتµؤء؟إ¨¶ب£¬½ّذذءثزشدآتµرé²ظ×÷£؛
A ہنب´ضءتزخآ؛َ£¬شع100mLبفء؟ئ؟ضذ¶¨بفإن³ة100mLد،ءٍثل،£
B ء؟ب،20.00mLد،ءٍثلسع׶ذخئ؟ضذ²¢µخب뼸µخض¸ت¾¼ء،£
C ½«ثلت½µخ¶¨¹ـ؛ح¼îت½µخ¶¨¹ـسأصôءَث®د´µس¸ة¾»£¬²¢سأ¸÷´ت¢بـز؛بَد´،£
D ½«خïضتµؤء؟إ¨¶بخھ1.50 mol،¤L£1µؤ±ê×¼NaOHبـز؛×°بë¼îت½µخ¶¨¹ـ£¬µ÷½عز؛أو¼ادآ¶ءتV1،£
E ¼جذّµخ¶¨ضءضصµم£¬¼ادآ¶ءتخھV2،£
F شع׶ذخئ؟دآµوز»صإ°×ض½£¬°ر׶ذخئ؟زئµ½¼îت½µخ¶¨¹ـدآذ،ذؤµخبëNaOH±ê×¼بـز؛£¬±كµخ±كز،¶¯×¶ذخئ؟،£
G ء؟ب،إ¨ءٍثلرùئ·5 mL£¬شعةص±ضذسأصôءَث®بـ½â،£
H ضط¸´زشةدتµرé،£
اë»ط´ًدآءذختجâ£؛
£¨1£©¸أتµرéصب·²ظ×÷²½ضèµؤث³ذٍخھ____،ْ A ،ْ_____،ْ_____،ْD،ْ_____،ْ_____،ْ H£¨سأ±à؛إ×ضؤ¸جîذ´£©،£
£¨2£©ء؟ب،5mLإ¨ءٍثلµؤزائ÷تا________________________£»ء؟ب،20.00mLد،ءٍثلµؤزائ÷تا_________،£
£¨3£©ر،سأµؤض¸ت¾¼ءتا_____________،£µخ¶¨¹³جضذ£¬تسدكس¦×¢تس____________________£»إذ¶دµ½´ïµخ¶¨ضصµمµؤدضدَتا________________£»¶ءتت±£¬تسدكس¦____________£¨جî،°¸كسع،±،¢،°µحسع،±»ٍ،°دàئ½سع،±£©بـز؛°¼ز؛أوµؤ×îµح´¦،£
£¨4£©دآ±يتاتµرé²âµأµؤسذ¹طت¾ف£؛
µخ¶¨ذٍ؛إ | ´²âد،ءٍثلµؤجه»(mL) | ثùدû؛ؤNaOH±ê×¼بـز؛ز؛µؤجه»(mL) | |
V1 | V2 | ||
¢ظ | 20£®00 | 0£®50 | 22£®60 |
¢ع | 20£®00 | 6£®00 | 27£®90 |
اë¼ئثم³ِ¸أإ¨ءٍثلرùئ·µؤإ¨¶بخھ____________mol،¤L£1 (²»±طذ´³ِ¼ئثم¹³ج)،£
،¾´ً°¸،؟D G C B F E ء؟ح² ثلت½µخ¶¨¹ـ ·سجھ»ٍ¼×»ù³ب ׶ذخئ؟ضذبـز؛رصة«µؤ±ن»¯ بـز؛سةخقة«±ن³ة؛ىة«£¨»ٍبـز؛سة؛ىة«±ن³ة³بة«£©از30sؤع²»حتة« دàئ½سع 16.5
،¾½âخِ،؟
¢ٌ. ¸ù¾فµخ¶¨¹ـ؟ج¶بضµ´سةدµ½دآ؟ج¶بضً½¥شِ´َزش¼°²âء؟شہي£¬×¢زâµخ¶¨¹ـ×î´َ؟ج¶بدآ·½خق؟ج¶ب£»
¢ٍ£®£¨1£©¸ù¾فضذ؛حµخ¶¨شہي؛حتµرéزھاَ½ّذذإإذٍ£»
£¨2£©ء؟ح²¾«ب·¶بخھ0.1mL£»µخ¶¨¹ـ¾«ب·¶بخھ0.01mL£»
£¨3£©¸ù¾فا؟ثلا؟¼îرخ³تضذذش£¬س¦ر،شٌثلذش»ٍ¼îذش·¶خ§ؤع±نة«µؤض¸ت¾¼ء£¬بç¼×»ù³ب»ٍ·سجھ£»¸ù¾فµخ¶¨¹³جضذ£¬ؤ؟¹âس¦×¢تس׶ذخئ؟ضذبـز؛رصة«µؤ±ن»¯£¬زش±م×¼ب·إذ¶دضصµمµؤµ½´ï£¬¶ءتت±س¦ئ½تسبـز؛°¼ز؛أوµؤ×îµح´¦£»
£¨4£©¼ئثمء½´ختµرéدû؛ؤاâرُ»¯ؤئµؤجه»£¬ب،ئ½¾ùضµ£¬زہ¾فثل¼îضذ؛ح·´س¦n£¨OH-£©=n£¨H+£©¼ئثمإ¨ءٍثلµؤخïضتµؤء؟إ¨¶ب،£
¢ٌ. µخ¶¨¹ـ؟ج¶بضµ´سةدµ½دآ؟ج¶بضً½¥شِ´َ£¬سةسعµخ¶¨¹ـ×î´َ؟ج¶بدآ·½خق؟ج¶ب£¬50mLµخ¶¨¹ـضذتµ¼تت¢·إز؛جهµؤجه»´َسع50ml£¬بç¹ûز؛أو´¦µؤ¶ءتتاa£¬شٍµخ¶¨¹ـضذز؛جهµؤجه»´َسع(50a)mL£¬¹تر،D،£
¢ٍ. (1)سأثلت½µخ¶¨¹ـ×¼ب·ء؟ب،إ¨ءٍثلرùئ·10.00mL£¬شعةص±ضذسأصôءَث®بـ½â£¬ہنب´ضءتزخآ؛َ,شع250mLبفء؟ئ؟ضذ¶¨بفإن³ة250mLد،ءٍثل£¬سأزئز؛¹ـزئب،25.00mLد،ءٍثلسع׶ذخئ؟ضذ²¢µخب뼸µخض¸ت¾¼ء£¬½«خïضتµؤء؟إ¨¶بخھM mol/Lµؤ±ê×¼NaOHبـز؛×°بë¼îت½µخ¶¨¹ـ,µ÷½عز؛أو,¼ادآ؟ھت¼¶ءتخھV1£¬شع׶ذخئ؟دآµوز»صإ°×ض½£¬µخ¶¨ضءضصµم,¼ادآ¶ءتV2£¬¹تصب·µؤ²ظ×÷ث³ذٍخھ£؛G،ْA،ْC،ْB،ْD،ْF،ْE،ْH£¬
¹ت´ً°¸خھ£؛G£»C£»B£»F£»E£»
(2)ء؟ح²¾«ب·¶بخھ0.1mL£»µخ¶¨¹ـ¾«ب·¶بخھ0.01 mL£¬ثùزشء؟ب،5mLإ¨ءٍثلµؤزائ÷£؛ء؟ح²£»ء؟ب،20.00mLد،ءٍثلµؤزائ÷تا£؛ثلت½µخ¶¨¹ـ£»¹ت´ً°¸خھ£؛ء؟ح²£»ثلت½µخ¶¨¹ـ£»
(3)ءٍثل؛حاâرُ»¯ؤئ·´س¦ةْ³ةءٍثلؤئ£¬ءٍثلؤئبـز؛³تضذذش£¬س¦ر،شٌثلذش»ٍ¼îذش·¶خ§ؤع±نة«µؤض¸ت¾¼ء£¬بç·سجھ»ٍ¼×»ù³ب£»شعµخ¶¨¹³جضذ£¬ؤ؟¹âس¦×¢تس׶ذخئ؟ضذبـز؛رصة«µؤ±ن»¯£»ءٍثلضذ¼سبë·سجھ£¬بـز؛دشت¾خقة«£¬ءٍثل·´س¦حêب«؛َ£¬¼سبëاâرُ»¯ؤئبـز؛؛َ£¬بـز؛دشت¾؛ىة«از°ë·ضضسؤع²»دûت§£»¶ءتت±£¬تسدكس¦دàئ½سعبـز؛°¼ز؛أوµؤ×îµح´¦£»¹ت´ً°¸خھ£؛·سجھ»ٍ¼×»ù³ب£»×¶ذخئ؟ضذبـز؛رصة«µؤ±ن»¯£»بـز؛سةخقة«±ن³ة؛ىة«£¨»ٍبـز؛سة؛ىة«±ن³ة³بة«£©از30sؤع²»حتة«£»دàئ½سع£»
(4)¢ظدû؛ؤاâرُ»¯ؤئµؤجه»خھ£؛22.600.50=22.10 (mL)£»
¢عدû؛ؤاâرُ»¯ؤئµؤجه»خھ£؛27.906.00=21.90 (mL)£»
شٍدû؛ؤاâرُ»¯ؤئبـز؛جه»µؤئ½¾ùضµخھ£؛(22.10+21.90) ،آ2 = 22.00 (mL)£¬
زہ¾فثل¼îضذ؛حµخ¶¨شہيn(OH)=n(H+)µأ1.50molL1،ء22.00mL = 20.00ml،ء2c(د،)£¬½âµأثùµأد،ءٍثلµؤإ¨¶بc(د،) = 0.825mol/L£¬شظ¸ù¾فد،تح¹وآةc(د،)V(د،)=c(إ¨)V(إ¨)؟ةضھ£¬¸أإ¨ءٍثلرùئ·µؤإ¨¶بخھc(إ¨)=![]() =16.5mol/L،£
=16.5mol/L،£



| ؤ꼶 | ¸كضذ؟خ³ج | ؤ꼶 | ³ُضذ؟خ³ج |
| ¸كز» | ¸كز»أâ·ر؟خ³جحئ¼ِ£، | ³ُز» | ³ُز»أâ·ر؟خ³جحئ¼ِ£، |
| ¸ك¶ | ¸ك¶أâ·ر؟خ³جحئ¼ِ£، | ³ُ¶ | ³ُ¶أâ·ر؟خ³جحئ¼ِ£، |
| ¸كب | ¸كبأâ·ر؟خ³جحئ¼ِ£، | ³ُب | ³ُبأâ·ر؟خ³جحئ¼ِ£، |
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟تµرéتز±¸سذضتء؟·ضتخھ98%,أـ¶بخھ1.84 g،¤cm-3µؤءٍثل,¾ف´ثدآءذثµ·¨´يخَµؤتا (،،،،)
A. ¸أءٍثلµؤخïضتµؤء؟إ¨¶بخھ18.4 mol،¤L-1
B. ¸أءٍثل50 mLسë×مء؟µؤح·´س¦؟ةµأµ½±ê×¼×´؟ِدآSO2 0.46 mol
C. ؤ³ح¬ر§سأ¸أءٍثلإنضئد،ءٍثلت±,خ´د´µسةص±؛ح²£ء§°ô,»لشى³ة×îضصإنضئµؤد،ءٍثلإ¨¶بئ«µح
D. µبضتء؟µؤث®سë¸أءٍثل»ى؛دثùµأبـز؛µؤخïضتµؤء؟إ¨¶بذ،سع9.2 mol،¤L-1
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟زرضھ£؛![]() +CH3CHO+HBr
+CH3CHO+HBr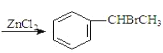 +H2O£¨آ±حé»ù»¯·´س¦£©;
+H2O£¨آ±حé»ù»¯·´س¦£©;
![]() +
+![]()
![]()
![]() +NaX
+NaX
سأ±½خھشءد؛د³ة»¯؛دخï¢ôµؤدكآ·بçدآ£؛
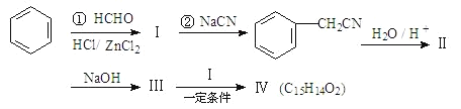
ئنضذ£؛¢ٍتاôبثل،£اë»ط´ًدآءذختجâ£؛
(1)¢عµؤ·´س¦ہàذحتا________،£
(2)ذ´³ِح¬ت±·û؛ددآءذجُ¼µؤ»¯؛دخï¢ٍµؤح¬·ضزى¹¹جه½ل¹¹¼ٍت½(ذ´2ضض)_____،¢_____،£
a.ؤـسëFeCl3بـز؛×÷سأدش×دة«£»
b.ؤـ·¢ةْزّ¾µ·´س¦£»
c.؛ث´إ¹²صٌاâئ׳±½»··هح⻹سذب×é·ه£¬·هأو»ض®±بخھخھ 1£؛2£؛1،£
(3)1mol»¯؛دخï¢ôحêب«ب¼ةصدû؛ؤO2_____mol£¬»¯؛دخï¢ôµؤ½ل¹¹¼ٍت½تا__________،£
(4)»¯؛دخï¢ٍسëزز´¼،¢إ¨ءٍثل¹²بب£¬؛د³ةز»ضضدم¾«شءد£¬تشذ´³ِ¸أ·´س¦µؤ»¯ر§·½³جت½__________،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
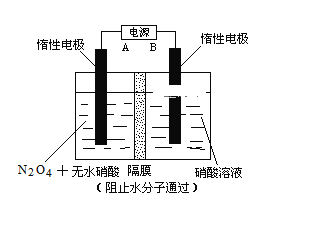
،¾جâؤ؟،؟N2O5تاز»ضضذآذحدُ»¯¼ء£¬ئنذشضت؛حضئ±¸تـµ½بثأاµؤ¹ط×¢،£زرضھN2O5ؤـسëث®·¢ةْ·´س¦£¬²¢·إ³ِ´َء؟µؤبب،£
¢ٌ.ز»¶¨خآ¶بدآ£¬شع؛مبفأـ±صبفئ÷ضذN2O5·¢ةْدآءذ·´س¦£؛2N2O5(g)![]() 4NO2(g)£«O2(g)¦¤H،£
4NO2(g)£«O2(g)¦¤H،£
£¨1£©دآ±يخھ·´س¦شعT1خآ¶بدآµؤ²؟·ضتµرéت¾ف£؛
t/s | 0 | 500 | 1000 |
c(N2O5)/mol،¤L،ھ1 | 5.00 | 3.52 | 2.48 |
شٍ500sؤعNO2µؤةْ³ةثظآتخھ______________،£
£¨2£©·´س¦´ïµ½ئ½؛â؛َ£¬بôشظح¨بëز»¶¨ء؟N2O5£¬´ïµ½ذآئ½؛âت±£¬N2O5µؤ×ھ»¯آت½«______£¨جî،°شِ´َ،±،¢،°¼ُذ،،±،¢،°²»±ن،±£©،£
£¨3£©شعخآ¶بT1؛حT2ت±£¬N2O5µؤإ¨¶بسë·´س¦ت±¼نµؤ¹طدµبçح¼ثùت¾،£¾ف´ثإذ¶د£؛T1______T2£¨جî،°>،±،¢،°<،±»ٍ،°=،±£¬دآح¬£©£¬¦¤H______0،£
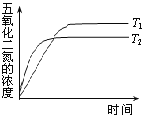
¢ٍ.دآح¼ثùت¾×°ضأ£¬؟ةسأسعضئ±¸N2O5ئّجه،£Aتاµçش´µؤ____________£¨جî،°¸؛¼«،±»ٍ،°ص¼«،±£©£¬µç½â³طµؤرô¼«·´س¦ت½خھ______________________________________________________،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟رذ¾؟¼ُةظCO2إإ·إتاز»دîضطزھ؟خجâ،£CO2¾´ك»¯¼ساâ؟ةزشةْ³ةµحج¼سذ»ْخض÷زھسذزشدآ·´س¦£؛
·´س¦¢ٌ£؛CO2(g)£«3H2(g) ![]() CH3OH(g)£«H2O(g) ،÷H1£½£49.6 kJ/mol
CH3OH(g)£«H2O(g) ،÷H1£½£49.6 kJ/mol
·´س¦¢ٍ£؛CH3OCH3(g)£«H2O(g) ![]() 2CH3OH(g) ،÷H2£½£«23.4 kJ/mol
2CH3OH(g) ،÷H2£½£«23.4 kJ/mol
·´س¦¢َ£؛2CO2(g)£«6H2(g) ![]() CH3OCH3(g)£«3H2O(g) ،÷H3
CH3OCH3(g)£«3H2O(g) ،÷H3
£¨1£©،÷H3£½________kJ/mol،£
£¨2£©؛مخآ؛مبفجُ¼دآ£¬شعأـ±صبفئ÷ضذح¨بëµبخïضتµؤء؟µؤCO2؛حH2£¬·¢ةْ·´س¦I،£دآءذأèتِؤـثµأ÷·´س¦I´ïµ½ئ½؛â×´ج¬µؤتا_______£¨جîذٍ؛إ£©،£
A£®·´س¦جهدµ×ـر¹ا؟±£³ض²»±ن
B£®بفئ÷ؤعµؤ»ى؛دئّجهµؤأـ¶ب±£³ض²»±ن
C£®ث®·ض×سضذ¶دءر2NA¸ِH-O¼ü£¬ح¬ت±اâ·ض×سضذ¶دءر3NA¸ِH-H¼ü
D£®CH3OH؛حH2Oµؤإ¨¶بض®±ب±£³ض²»±ن
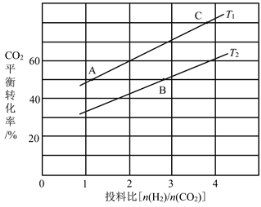
£¨3£©·´س¦IIشعؤ³خآ¶بدآµؤئ½؛â³£تخھ0.25£¬´ثخآ¶بدآ£¬شعأـ±صبفئ÷ضذ¼سبëµبخïضتµؤء؟µؤCH3OCH3(g)؛حH2O(g)£¬·´س¦µ½ؤ³ت±؟ج²âµأ¸÷×é·ضإ¨¶ببçدآ£؛
خïضت | CH3OCH3(g) | H2O(g) | CH3OH(g) |
إ¨¶ب/mol،¤L£1 | 1.8 | 1.8 | 0.4 |
´ثت±vص___vؤو£¨جî،°£¾،±،¢،°£¼،±»ٍ،°£½،±£©£¬µ±·´س¦´ïµ½ئ½؛â×´ج¬ت±£¬»ى؛دئّجهضذCH3OHجه»·ضت(CH3OH)% £½___%،£
£¨4£©شعؤ³ر¹ا؟دآ£¬·´س¦IIIشع²»ح¬خآ¶ب،¢²»ح¬ح¶ءد±بت±£¬CO2µؤئ½؛â×ھ»¯آتبçح¼ثùت¾،£T1خآ¶بدآ£¬½«6mol CO2؛ح12mol H2³نبë2 Lµؤأـ±صبفئ÷ضذ£¬5min؛َ·´س¦´ïµ½ئ½؛â×´ج¬£¬شٍ0،«5minؤعµؤئ½¾ù·´س¦ثظآتv(CH3OCH3)£½____£»KA،¢KB،¢KCبصكض®¼نµؤ´َذ،¹طدµخھ____،£
£¨5£©؛مر¹دآ½«CO2؛حH2°´جه»±ب1£؛3»ى؛د£¬شع²»ح¬´ك»¯¼ء×÷سأدآ·¢ةْ·´س¦I؛ح·´س¦III£¬شعدàح¬µؤت±¼ن¶خؤعCH3OHµؤر،شٌذش؛ح²ْآتثوخآ¶بµؤ±ن»¯بçح¼،£ئنضذ£؛CH3OHµؤر،شٌذش£½![]() ،ء100%
،ء100%

شعةدتِجُ¼دآ؛د³ة¼×´¼µؤ¹¤زµجُ¼تا____،£
A£®210،و B£®230،و C£®´ك»¯¼ءCZT D£®´ك»¯¼ءCZ(Zr£1)T
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟دآءذض¸¶¨·´س¦µؤہë×س·½³جت½صب·µؤتا (،،،،)
A. FeOبـسعد،دُثل:FeO+2H+ ![]() Fe2++H2O
Fe2++H2O
B. Al2(SO4)3بـز؛ضذ¼سبë¹ء؟Ba(OH)2بـز؛:Al3++4OH- ![]() AlO2-+2H2O
AlO2-+2H2O
C. ئ¯°×·غبـز؛ضذح¨بëةظء؟CO2: 2ClO-+H2O+CO2![]() 2HClO+CO32-
2HClO+CO32-
D. دٍNaOHبـز؛ضذµخ¼س¹ء؟Ca(HCO3)2بـز؛:Ca2++HCO3-+OH-![]() CaCO3،+H2O
CaCO3،+H2O
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟ؤ³شھثطµؤز»¸ِش×سذخ³ةµؤہë×س؟ة±يت¾خھ![]() n-£¬دآءذثµ·¨صب·µؤتا£¨ £©
n-£¬دآءذثµ·¨صب·µؤتا£¨ £©
A.![]() n-ضذ؛¬سذµؤضذ×ستخھa+b
n-ضذ؛¬سذµؤضذ×ستخھa+b
B.![]() n-ضذ؛¬سذµؤµç×ستخھa-n
n-ضذ؛¬سذµؤµç×ستخھa-n
C.Xش×سµؤضتء؟تخھa+b+n
D.ز»¸ِXش×سµؤضتء؟ش¼خھ![]() g
g
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟CH4£¨ئنضذCخھ£4¼غ£©¼بتاز»ضضضطزھµؤؤـش´£¬ز²تاز»ضضضطزھµؤ»¯¹¤شءد،£
£¨1£©زرضھ8.0gCH4حêب«ب¼ةصةْ³ة¶رُ»¯ج¼؛حز؛ج¬ث®ت±·إ³ِ444.8kJببء؟،£بôز»¶¨ء؟µؤ¼×حéحêب«ب¼ةصةْ³ة¶رُ»¯ج¼؛حز؛ج¬ث®ت±·إ³ِ1334.4kJµؤببء؟£¬شٍذèزھ±ê×¼×´؟ِدآµؤرُئّش¼___L،£
£¨2£©زشCH4خھب¼ءد؟ةةè¼ئ³ة½ل¹¹¼ٍµ¥،¢ؤـء؟×ھ»¯آت¸ك،¢¶ش»·¾³خقخغب¾µؤب¼ءدµç³ط£¬ئن¹¤×÷شہيبçح¼¼×ثùت¾£¬ح¨بë2.24L£¨زر»»ثمخھ±ê×¼×´؟ِ£©aئّجهت±£¬ح¨¹ضت×س½»»»ؤ¤×ھزئµؤH+تؤ؟خھ___£¨ةèNAخھ°¢·ü¼سµآآق³£تµؤضµ£©،£

£¨3£©شعز»¶¨خآ¶ب؛ح´ك»¯¼ء×÷سأدآ£¬CH4سëCO2؟ةض±½س×ھ»¯³ةززثل£¬صâتاتµدض،°¼ُإإ،±µؤز»ضضرذ¾؟·½دٍ،£
¢ظشع²»ح¬خآ¶بدآ£¬´ك»¯¼ءµؤ´ك»¯ذ§آتسëززثلµؤةْ³ةثظآتبçح¼ززثùت¾£¬شٍ¸أ·´س¦µؤ×î¼رخآ¶بس¦؟طضئشع___،و×َسز،£
¢ع¸أ·´س¦´ك»¯¼ءµؤسذذ§³ة·ضخھئ«آءثلراح(CuAlO2£¬ؤربـخï)،£½«CuAlO2بـ½âشعد،دُثلضذةْ³ةء½ضضرخ²¢·إ³ِNOئّجه£¬ئن·´س¦µؤہë×س·½³جت½خھ___،£
¢غCH4»¹ش·¨تا´¦ہيNOxئّجهµؤز»ضض·½·¨،£زرضھز»¶¨جُ¼دآCH4سëNOx·´س¦×ھ»¯خھN2،¢CO2؛حH2O£¬بô±ê×¼×´؟ِدآ8.96LCH4؟ة´¦ہي22.4LNOx£¬شٍxضµخھ___،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟A،¢B،¢C،¢D،¢E ¾ùخھسذ»ْخئنضذ B تا»¯ر§تµرéضذ³£¼ûµؤسذ»ْخ ثüز×بـسعث®²¢سذجطتâدمخ¶£»A µؤ²ْء؟؟ة؛âء؟ز»¸ِ¹ْ¼زت¯سح»¯¹¤·¢ص¹µؤث®ئ½£¬G تاةْ»îضذ³£¼ûµؤ¸ك·ض×س²ؤءد،£سذ¹طخïضتµؤ×ھ»¯¹طدµبçح¼¼×ثùت¾£؛
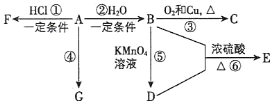
(1)ذ´³ِ A µؤ½ل¹¹ت½_____£»B ضذ¹ظؤـحإµؤأû³ئخھ_____،£
(2)ذ´³ِدآءذ·´س¦µؤ»¯ر§·½³جت½£؛
·´س¦¢غ____£»
·´س¦¢ـ____،£
£¨3£©تµرéتزہûسأ·´س¦¢قضئب، E£¬³£سأبçح¼×°ضأ£؛
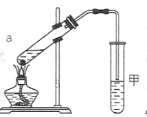
¢ظa تش¹ـضذض÷زھ·´س¦µؤ»¯ر§·½³جت½خھ_____،£
¢عتµرé؟ھت¼ت±£¬تش¹ـ¼×ضذµؤµ¼¹ـ²»ةىبëز؛أودآµؤشزٍتا_____£»µ±¹غ²ىµ½تش¹ـ¼×ضذ_____ت±£¬بدخھ·´س¦»ù±¾حê³ة،£
²é؟´´ً°¸؛ح½âخِ>>
¹ْ¼تر§ذ£سإر، - ء·د°²لءذ±ي - تشجâءذ±ي
؛±±ت،»¥ءھحّخ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨ئ½ج¨ | حّةدسذ؛¦ذإد¢¾ظ±¨×¨اّ | µçذإص©ئ¾ظ±¨×¨اّ | ةوہْت·ذéخقض÷زهسذ؛¦ذإد¢¾ظ±¨×¨اّ | ةوئَاضب¨¾ظ±¨×¨اّ
خ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨µç»°£؛027-86699610 ¾ظ±¨ستدن£؛58377363@163.com