
����Ŀ��(1)�Ȼ�����Һ���ڼ���ʳ���㾫��������ʱ����������ɫ�����������ӽṹ��ͼ��ʾ��
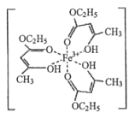
�ٴ�������У���̬�����ӵļ۵����Ų�ʽΪ________________��
�ڴ��������̼ԭ�ӵ��ӻ����������________________��
�۴��������к��еĻ�ѧ����____________(����ĸ)��
A. ���Ӽ� B. ������ C. ���Լ� D. �Ǽ��Լ� E. ��λ�� F. ��� G.���� H. ����
���Ȼ����ڳ������ǹ��壬�۵�Ϊ 306�����е�Ϊ 315������ 300������������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж��Ȼ����ľ�������Ϊ_________��
(2)��̬ A ԭ�ӵļ۵����Ų�ʽΪ 3s23p5��ͭ��A �γɻ�����ľ�����ͼ��ʾ(�������ͭԭ��)��
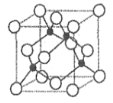
�ٸû�����Ļ�ѧʽΪ____________��Aԭ�ӵ���λ����______________��
����֪�û����ᄃ����ܶ�Ϊ��g��cm-3�������ӵ�������ֵΪ NA����þ����� Cu ԭ�Ӻ�A ԭ��֮�����̾���Ϊ________pm(�г��������ʽ����)��
���𰸡�3d5 sp3 sp2 C D E G H ���Ӿ��� CuCl 4 
��������
(1)�������֪���������е�FeΪ+3�ۣ������۵����Ų�ʽΪ3d5��
�������֪����������еĵ������е�Cԭ�����γ�4�������ģ�Ҳ���γ�˫���ģ�����ӻ���ʽ��sp3��sp2��
����������������ԭ�Ӻ�����ͨ����λ���γɵģ���ͼ��֪�����������к���λ�����Լ������ڲ����ڵļ��Լ����Ǽ��Լ����Ҽ��Լ��м�������CDEGH��ȷ��
�������֪��FeCl3�����۷е㲻�ߣ��������������������л��ܼ��е����ʣ������Ʋ���Ϊ���ۻ�������ڷ��Ӿ��壻
(2)�ɻ�̬Aԭ�ӵļ۵����Ų�ʽ��֪AΪClԪ�أ�
���ɾ����ṹ��֪��Cuλ�ھ������ڲ���һ�������к���![]() ��Cu��Clλ�ھ��������ĺͶ��㴦��һ�������к���
��Cu��Clλ�ھ��������ĺͶ��㴦��һ�������к���![]() ��Cl�����Ծ�����Cu��Cl�ĸ�����Ϊ1:1���û����ﻯѧʽΪCuCl���ɾ����ṹ��֪��Cl-��Χ�����Cu+��4��������Cl-����λ��Ϊ4��
��Cl�����Ծ�����Cu��Cl�ĸ�����Ϊ1:1���û����ﻯѧʽΪCuCl���ɾ����ṹ��֪��Cl-��Χ�����Cu+��4��������Cl-����λ��Ϊ4��
���ɾ����ṹ��֪��Cu+��Cl-����̾��뼴Ϊ������Խ��ߵ�![]() �������֪�������ı߳�aΪ��
�������֪�������ı߳�aΪ��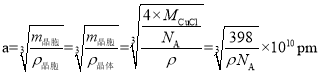 ����ôCu+��Cl-����̾��뼴
����ôCu+��Cl-����̾��뼴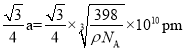 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼװ�ñպϵ��Kʱ��������A��ָ�뽫����ƫת���Իش�

(1) ������____________������ԭ�����������������������a���缫������________________������c���缫������_____________________��
(2)����Cu���ĵ缫��Ӧʽ��__________������·����0.02mol����ͨ���������a�缫�ܽ������Ϊ__________g��
(3)�պϵ��Kһ��ʱ��������������������һ�ּ������з������ܵĻ�ѧ����ʽ��_________________��
��4�������з�Ӧ���нϳ�ʱ����ռ�����״��������2.24L����ʱ��ñ�����Һ����ʵ�ʼ���4.23 g�����м�0.100mol��������������ˮ�е��ܽ⣩����ʵ�ʷų���������ʵ�����______________mol��
��5�����Ҫ��������Ƭ�϶���һ��Cu�������Ӧ���θĽ�_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±�Ϊ��ʽ���ڱ���һ�������еı�Ŵ�����Ӧ��Ԫ��.
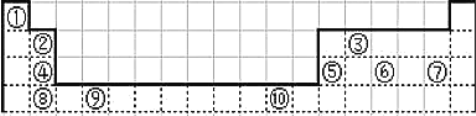
��ش��������⣺
(1)�������� ds ����Ԫ����___________(��Ԫ�ط���)���Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ___________��
(2)����Ԫ�آٵ� 6 ��ԭ����Ԫ�آ۵� 6 ��ԭ���γɵ�ij��ƽ�滷״���������ʽΪ____________������Ԫ�آ۵Ļ�̬ԭ�ӹ������ʽΪ___________���ٺ͢��γɵ�һ�ֳ�����ԭ�ӷ��ӵĻ�ѧʽΪ___________������ _________________(���������������Ǽ�����)���ӡ�
(3)ijԪ��ԭ�ӵļ۵����Ų�ʽΪ nsnnpn+1����Ԫ��ԭ�ӵĵ��Ӳ���δ�ɶԵ�����Ϊ___________����Ԫ����Ԫ�آ��γɵ������ X �ĵ���ʽΪ___________��
(4)Ԫ�آݵĵ縺��___________��Ԫ�صĵ縺��(ѡ��>��=��<��ͬ)��Ԫ�آĵ�һ������___________ԭ������Ϊ 16 ��Ԫ�صĵ�һ�����ܡ�
(5)�õ���ʽ��ʾԪ�آܺ͢���ɵĻ�������γɹ���________________________��
(6)�ϱ���Ԫ�آݵ���������Ϊ�������������д��Ԫ�آݵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��³ʿ���Ļ�ѧʽ��KFe[Fe(CN)6]�������ʵĻ�ѧ�������Ӽ������ۼ���_____��
(2)KOCN�����Ӿ��壻̼ԭ�Ӳ�ȡsp�ӻ���1mol�������к��е�������ĿΪ____��
(3)H2O2��������Һ�壬�е�ϸ�(150��)������Ҫԭ����____��
(4)�ǽ���Ԫ��![]() �ĵ�һ�����ܴ���
�ĵ�һ�����ܴ���![]() �ĵ�һ�����ܣ�ԭ����______��
�ĵ�һ�����ܣ�ԭ����______��
(5)V2O5����NaOH��Һ���ɵõ�������(Na3VO4)�����������ӵ����幹��Ϊ________��
(6)��֪ʳ�ε��ܶ�Ϊ�� g��cm��3����Ħ������ΪM g��mol��1�������ӵ�����ΪNA������ʳ�ξ��徧��������_______cm��
(7)1 mol SiO2�����к�________ mol Si��O����
(8)1 mol NH4BF4����________ mol�����
(9)�������ʯ��MgO��CaCl2���ɱ�5�־�����۵��ɸߵ��͵�˳��Ϊ��_____________��
(10)���������ṹ�Ľ����磺Na��K��Fe�ľ����Ŀռ�ռ���ʱ���ʽΪ________(����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȷ��ʾ���з�Ӧ�����ӷ���ʽ����
A. ��Na2SiO3��Һ�м������2Na++ SiO32- +2H+![]() H2SiO3��+2Na+
H2SiO3��+2Na+
B. �������ռ���Һ��Ӧ��Cl2+2OH-![]() Cl-+ClO-+H2O
Cl-+ClO-+H2O
C. ϡ���������Ƭ�ϣ�Fe+6H+![]() 2Fe3++3H2��
2Fe3++3H2��
D. ͭƬ������������Һ�У�Cu+Ag+![]() Cu2++Ag
Cu2++Ag
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��(Mo)�ľ�����ͼ��ʾ����ԭ�Ӱ뾶Ϊapm�����ԭ������ΪM����NA��ʾ�����ӵ�������ֵ��
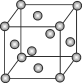
��1���⾧��Ķѻ���ʽΪ__��
��2����ԭ�ӵ���λ��Ϊ__��
��3�������⾧���������__��
��4����������ܶ�Ϊ__g��cm-3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2mol/L CuCl2��Һ��2mol/L����������ϣ������Ϻ���Һ������ڻ��ǰ������Һ�����֮�ͣ�������㣺
��1�����Һ��CuCl2����������ʵ���Ũ�ȣ�_______________
��2�����Һ��H����Cu2����Cl�������ʵ���Ũ�ȣ�______________
��3������Һ�м������������ۣ����㹻����ʱ���������ʣ�ࡣ��ʱ��Һ��FeCl2�����ʵ���Ũ�ȡ�____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������зḻ��ʳƷ���������Դ��ҩ���ˮ����Դ��(��ͼ��ʾ)��

�����й�˵����ȷ����
A.�������г�ȥ�����е�![]() ��Ca2+��Mg2+��Fe3+�����ʣ������ҩƷ˳��Ϊ�� Na2CO3��Һ��BaCl2��Һ��NaOH��Һ�����������������pH
��Ca2+��Mg2+��Fe3+�����ʣ������ҩƷ˳��Ϊ�� Na2CO3��Һ��BaCl2��Һ��NaOH��Һ�����������������pH
B.MgSO4��7H2O�ڿ����м��ȿ��Ƶ���ˮMgSO4�ķ�������������ơ�
C.�ӵ���������������Ŀ����Ϊ��Ũ������Br2
D.�ڵ��ۢܢ�������Ԫ�ؾ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ���������ֵ������˵����ȷ����(����)
A.1mol�һ��к��еĵ�����Ϊ16NA
B.8g CH4���10NA������
C.��״����22.4L�ȷ��й��ۼ���ĿΪ4NA
D.28g��ϩ����ϩ����ϩ�Ļ�����壬��̼ԭ����Ϊ2NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com