
����Ŀ��A��B��C��D��E��Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ���ԭ��������������AΪԪ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����ռ�е��ӵ�3���ܼ��ϵĵ���������ȣ�D��Bͬ�壬C��Bͬ���ڣ���C������p����ϵĵ�����������s����ϵĵ�������ȣ�E�Ĵ��������������������ӵ�7����
�ش��������⣺
(1)B��C��D����Ԫ�صĵ縺����С�����˳��Ϊ___________��(��Ԫ�ط��ű�ʾ)��DԪ�ػ�̬ԭ�Ӽ۲�����Ų�ʽΪ______________________��
(2)A��C�γɵ���ԭ�ӷ����У�Cԭ�ӵ��ӻ���ʽΪ___________��
(3)C��D�γɵĻ�����ľ�������Ϊ___________��
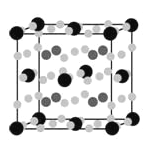
(4)����Mg��A��E�γɵĻ�������Ŀǰ�����ѷ��ֵ���������ܶ���ߵĴ������֮һ���侧���ṹ��ͼ��ʾ�����к������E���������Mg���������A�����а���������ϡ��������⣬�ھ����ڲ�����6������д���û�����Ļ�ѧʽ��______________________��
(5)B��C��E���γ���ͼ��ʾ����˫���͵��������ӣ�����Ԫ�ص�ԭ�ӷֱ��ô����С����ͺ��������
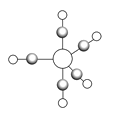
��������γ���λ��ʱ�ṩ�չ����ԭ����___________(��Ԫ�ط���)����������д���������Ԫ�صĻ��ϼ�Ϊ___________��
����ˮ��Һ�У�ˮ�Զ���������ʽ�������������γ�ˮ����Ի����������Ľṹͼʽ��H5O2+��______________________
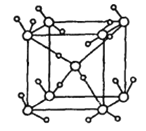
����ͼΪ����һ�ֹǼ���ʽ������Ϊ��λ��ռ�����ñ��е�ÿ��ˮ������___________���������������Ǿ���ͼ������ͣ���һ���ʵĿռ����ӷ�ʽ�����ֱ�����������______________________
���𰸡�Si <C<0 3s 2 3p 2 sp 3 ԭ�Ӿ��� Mg2FeH6 Fe 0 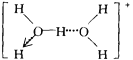 2 SiO2
2 SiO2
��������
����Ԫ�����ڱ��Ų������ж�Ԫ�����ࣻ���ݼ۲���ӶԻ������۷�����𣻸��ݾ���Ľṹ�����ʷ������
AΪԪ�����ڱ���ԭ�Ӱ뾶��С��Ԫ����A��HԪ�أ�B�Ļ�̬ԭ����ռ�е��ӵ�3���ܼ��ϵĵ���������ȣ���B��CԪ�أ�D��Bͬ�弴D��SiԪ�أ�C��Bͬ���ڼ�CԪ��Ϊ��ڶ����ڣ���C������p����ϵĵ�����������s����ϵĵ�������ȣ���C�Ļ�̬ԭ�Ӻ�������Ų�Ϊ��1s22s22p4��C��OԪ�أ�E�Ĵ��������������������ӵ�7����E�Ļ�̬ԭ�Ӻ�������Ų�Ϊ��1s22s22p63s23p63d64s2������E��FeԪ�ء�
(1) ͬһ���ڣ�������Ԫ�ص縺�Ե�����ͬһ���壬���϶���Ԫ�ص縺�Եݼ������縺�ԣ�Si<C<O��D��SiԪ�أ����Ļ�̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p2�������Ļ�̬ԭ�Ӽ۲�����Ų�ʽΪ��3s23p2��
�ʴ�Ϊ��Si<C<O��3s23p2��
(2) A��HԪ�أ�C��OԪ�أ������γɵ���ԭ�ӷ�����H2O�����ݼ۲���ӶԻ�������,�ĶԵ�����ų��������ṹ,�ɼ��ĸ�������������ӻ�,������sp3�ӻ����ʴ�Ϊ��sp3��
(3)C��OԪ�أ�D��SiԪ�أ��γɻ�����SiO2��Si��O���ǹ��ۼ���SiO2����ԭ�Ӿ��壻
�ʴ�Ϊ��ԭ�Ӿ��壻
(4) 8��Mgԭ���ھ����ڲ�������ΪFeԭ�ӣ�һ�������к��е�Feԭ����Ϊ8��![]() +6��
+6��![]() =4��aΪHԭ�ӣ�һ�������к��е�Hԭ����Ϊ6+(12��2)��
=4��aΪHԭ�ӣ�һ�������к��е�Hԭ����Ϊ6+(12��2)��![]() +(4��6)��
+(4��6)��![]() =24��Mg��Fe��H=2��1��6���������ʽΪMg2FeH6��
=24��Mg��Fe��H=2��1��6���������ʽΪMg2FeH6��
�ʴ�Ϊ��Mg2FeH6��
(5) )����˫����Ϊ�Գ��Խṹ����˸÷���Ϊ�Ǽ��Է��ӣ��Ա��������ԭ�Ӱ뾶��С��֪С����ΪOԭ�ӣ�����ΪCԭ�ӣ������ΪFeԭ�ӣ�Feԭ�Ӿ��ж����ԭ�ӹ�������ṩ�չ�����γɻ�����Fe(CO)5������Fe(CO)5��Fe�Ļ��ϼ�Ϊ0��
�ʴ�Ϊ��Fe��0��
�� H5O2+����д������ˮ�������������������һ��ˮ���ӵ�O�����������һ�������ӣ���дʱ������ԭ�ӿ�Ϊһ�������÷���������������+�����ڷ��������Ͻǣ����ṹΪ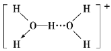 ��
��
�ʴ�Ϊ��![]() ��
��
��������У�ÿ����ˮ���Ӽ���һ�������ƽ������ÿ��ˮ������0.5����һ��ˮ��������Χ���ĸ�ˮ�����������ϣ���1mol������2mol���������������ԭ�Ӿ���,SiOͨ�����ۼ��γ�������ṹ,������֮��ͨ�����ۼ��γɿռ���״�ṹ,���������е�ˮ����Ϊ�о�����,ÿ��ˮ�����γ�4�����, SiO2�ռ����ӷ�ʽ�����ֱ��������ƣ�
�ʴ�Ϊ��2��SiO2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ֵ��ָһ�������µ�λ������������ȫȼ�����ų�����������֪H2(g)��CO(g)��CH3OH(l)����ֵ�ֱ�Ϊ143 kJ�� g��1��10 kJ��g��1��23 kJ��g��1����ش��������⣺
(1)д��COȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ__________________________________________��
(2)��ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ__________________________________________��
(3)�״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ__________________________��
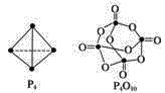
(4)���������ɷ������·�Ӧ��P4��g��+5O2(g)![]() P4O10(g)����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��P a kJ/mol��P��O b kJ/mol��P=O c kJ/mol��O=O d kJ/mol����ͼʾ�ķ��ӽṹ���й����ݼ���÷�Ӧ�ķ�Ӧ��_____________��
P4O10(g)����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��P a kJ/mol��P��O b kJ/mol��P=O c kJ/mol��O=O d kJ/mol����ͼʾ�ķ��ӽṹ���й����ݼ���÷�Ӧ�ķ�Ӧ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������Ҫ��ʩ�л�������ʱ��ͨ���ơ�������ҵͣҵ�����ﳾ��Ⱦ���Ƶȡ�
��1��PM2.5�ǻ������ż�������������Ҫָ�ꡣ��ijPM2.5����������ˮ�����Ƴɴ������������������������(OH�����Բ���)�������ƽ��Ũ�����±���
�������� | Na�� | NH4+ | SO42- | NO3- |
Ũ��(mol/L) | 2.0��10��6 | 2.8��10��5 | 3.5��10��5 | 6.0��10��5 |
��������pHΪ________________________��
��2��һ�������£���CO��H2�ϳ������ԴCH3OH�����Ȼ�ѧ����ʽΪCO(g)��2H2(g) CH3OH(g)����H��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

�ٸÿ��淴Ӧ�Ħ�H__________0(�>����<������)��A��B��C�����Ӧ��ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵ��______________��ѹǿ��p1__________p2(�>����<������)����T1�����£���D�㵽B������У������淴Ӧ����֮��Ĺ�ϵ��v(��)___________v(��)(�>����<������)��
�����ں��º��������½���������Ӧ���ܱ�ʾ�ÿ��淴Ӧ�ﵽƽ��״̬����_________(����ĸ)��
A.CO������������ֲ���
B�������ڻ��������ܶȱ��ֲ���
C�������ڻ�������ƽ��Ħ���������ֲ���
D����λʱ��������CO��Ũ�ȵ�������CH3OH��Ũ��
�����ѹ�ܱ������г���2 mol CO��4 mol H2����p2��T2�����´ﵽƽ��״̬C�㣬��ʱ�����ݻ�Ϊ2 L�����ڸ������·�Ӧ��ƽ�ⳣ��KΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£�CO��CO2�Ļ�����干39.2 L������Ϊ61 g����������������ʵ���֮��Ϊ__________ mol������CO2Ϊ__________mol��COռ�������__________����������Ħ������Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NiSO4��6H2O��һ����ɫ������ˮ�ľ��壬�㷺���ڻ�ѧ������������صȡ����ɵ�Ʒ���(�������⣬������Cu��Zn��Fe��Cr������)Ϊԭ�ϻ�á�������������ͼ��
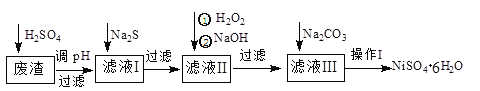
��ش��������⣺
(1)��ϡ�����ܽ����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ��_________________(��дһ��)��
(2)����Һ�е���������Na2S��Һ��Ŀ���dz�ȥCu2+��Zn2+��д����ȥCu2+�����ӷ���ʽ��____________________________________________��
(3)��40�����ң���6%��H2O2����Fe2+������95��ʱ����NaOH����pH����ȥ���������⣬������NaClO3�����������ڽ�С��pH������ˮ�⣬��������һ��dz��ɫ�Ļ�������[Na2Fe6(SO4)4(OH)12]������ȥ����ͼ���¶ȡ�pH�����ɵij�����ϵͼ��ͼ����Ӱ�����ǻ������ȶ����ڵ�����[��֪25��ʱ��Fe(OH)3��Ksp=2.64��10��39]��
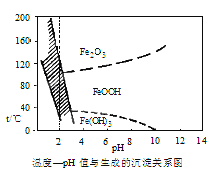
����˵����ȷ����(ѡ�����)_______��
a. FeOOH����Ϊ+2��
b.����25��ʱ����H2O2����Fe2+������pH=4ʱ��ȥ������ʱ��Һ��c(Fe3+)=2.6��10��29mol��L��1
c.������������������������Fe2+�����ӷ���ʽΪ6Fe2++C1O3��+6H+=6Fe3++C1��+3H2O
d.��ҵ�����г�������85~95�����ɻ������ƣ���ʱˮ���pHԼΪ3
(4)������������ҺI����Ҫ�ɷ���___________��
(5)����I��ʵ�鲽������Ϊ(ʵ���п�ѡ�õ��Լ���6mol��L��1��H2SO4��Һ������ˮ��pH��ֽ)��
��___________����___________��������Ũ������ȴ�ᾧ�����˵�NiSO4��6H2O���壺���������Ҵ�ϴ��NiSO4��6H2O���岢���ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£���һ���ݻ�Ϊ2 L���ܱ�������(Ԥ�ȼ������)ͨ��2 mol NH3������һ��ʱ���ƽ���������ڵ�ѹǿΪ��ʼʱ��1.2������NH3��ת����Ϊ
A. 25% B. 80% C. 10% D. 20%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ֱ����0.01mol��L��1HCOOH��Һ��0.01mol��L��1��ˮ��pH��ϵͳ����Ũ�ȵĸ�����ֵ(��lgc)��pH�Ĺ�ϵ�ֱ�����ͼ��
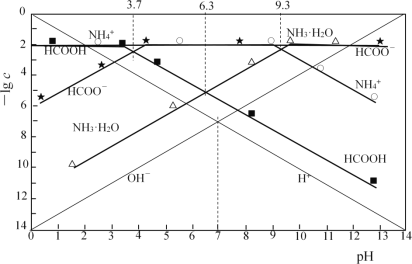
����˵����������
A. 25��ʱ��NH3��H2O![]() NH4++OH����lgK=��4.7
NH4++OH����lgK=��4.7
B. 25��ʱ��0.01mol��L��1HCOOH��Һ��pHΪ3.7
C. HCOONa��Һ�м���NH4Cl����Һ�����ԣ�c(Cl��)��c(Na+)��c(HCOO��)
D. HCOONa��Һ�м���KHSO3����Һ�����ԣ�c(HCOOH) +c(H2SO3) = c(SO32��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ca(NO2)2(�������)��������ˮ����ɫ���壬�������������иֽ�ķ�������
(1)Ca(NO2)2���Ʊ������ܶࡣ
��ʵ���ҿ��÷�ӦCa(NO3)2+2CaFe2O4+4NO![]() 3Ca(NO2)2+2Fe2O3�Ʊ�Ca(NO2)2���÷�Ӧ�б�������Nԭ���뱻��ԭ��Nԭ�ӵ����ʵ���֮��Ϊ_____��
3Ca(NO2)2+2Fe2O3�Ʊ�Ca(NO2)2���÷�Ӧ�б�������Nԭ���뱻��ԭ��Nԭ�ӵ����ʵ���֮��Ϊ_____��
����ʯ�����������Ṥҵβ���е��������Ʊ�Ca(NO2)2������NO2��Ca(OH)2��Ӧ����Ca(NO2)2��Ca(NO3)2�Ļ�ѧ����ʽΪ_____�������˵õ���Ca(NO2)2����ҺΪҺ̬��Ʒ��
(2)�ⶨijҺ̬��Ʒ��NO3�������IJ������£�
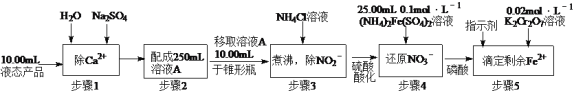
��֪������4�еķ�ӦΪNO3��+3Fe2++4H+=3Fe3++NO��+2H2O��
����5�еķ�ӦΪ6Fe2++Cr2O72��+14H+=6Fe3++2Cr3++7H2O��
������5�ζ����յ�ʱ����K2Cr2O7��Һ20.00mL������Һ̬��Ʒ��NO3���ĺ���(��λg��L��1�����������һλС����д���������)___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����CO�ϳɼ״�(CH3OH)�Ļ�ѧ��Ӧ����ʽΪCO(g)+2H2(g)![]() CH3OH(g) ��H < 0��������ͬ�����ʵ���Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
CH3OH(g) ��H < 0��������ͬ�����ʵ���Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����

A. ����Ӧ���ʣ�v(a)>v(c) v(b)>v(d)
B. ƽ��ʱa��һ����n(CO):n(H2)��1:2
C. ƽ�ⳣ����K(a)>K(c) K(b)��K(d)
D. ƽ��Ħ��������![]() (a)<
(a)<![]() (c)
(c)![]() (b)>
(b)>![]() (d)
(d)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com