
����Ŀ����ҩҩ����˪����Ҫ�ɷ�As2O3������ˮ����ҽ�����������Ƽ���Ѫ����ij��������һ�ֺ��龫��ʯ�ۣ���Ҫ�ɷ�ΪAs4S4��As2S3��FeS2�������������ʣ�Ϊԭ����ȡAs2O3, �������̼�ͼ���£�

�ش��������⣺
(1)����I������SO2����ɻ��������ã������й�SO2��;��˵����ȷ����______��
A.��ҵ������ B.Ư��ֽ�� C.����ˮ����
(2)����II�з����ķ�Ӧ______������ԭ��Ӧ����ǡ����ǡ�)��
(3)����V��ϵ�в���Ϊ_______ (�ѧʵ������������ƣ���
(4)�ٹ���I�б���As2S3�Ļ�ѧ��Ӧ����ʽΪ_______________��
�ڹ���IV������As2O3�����ӷ�Ӧ����ʽΪ_______________��
(5)�ж���AsO33-ͨ����ⷴӦ��ת��Ϊ����AsO43-����ʯīΪ�缫����ǿ������Һ��
��⺬AsO33-����Һ�������ĵ缫��ӦʽΪ______________��
(6)�ⶨijAs2O3�ֲ�Ʒ����As2O5���ʣ���As2O3������������ʵ��������£�
a.��ȡm g�ֲ�Ʒ�ܽ���NaOH��Һ���õ���AsO33-��AsO43-�Ļ����Һl00mL��
b.�ֱ���ȡ25.00mL������Һ����0.02500 mol��L-1��I2����Һ���еζ���I2��AsO33-����ΪAsO43-��������ҺΪָʾ������ÿ�εζ���ʼʱҺ�������ͼһ��ʾ�����εζ�����ʱ��I2����ҺҺ�������ͼ��ͼ����ʾ��
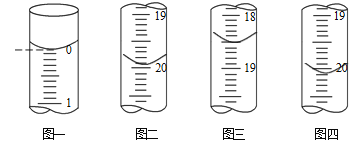
�������ζ��յ������_____________________��
�ڴֲ�Ʒ��As2O3����������Ϊ______________ (�ú���m�Ĵ���ʽ��ʾ����
���𰸡� AB ���� ���ˡ�ϴ�ӡ����� 2As2S3+9O2![]() 2As2O3+ 6SO2 2AsO43-+2SO2+2H+=As2O4��+2SO42-+H2O AsO33-+2OH--2e- = AsO43-+ H2O �����һ��I2����Һ����ʱ����Һ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٷ����仯�� (0.198/m)��100%
2As2O3+ 6SO2 2AsO43-+2SO2+2H+=As2O4��+2SO42-+H2O AsO33-+2OH--2e- = AsO43-+ H2O �����һ��I2����Һ����ʱ����Һ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٷ����仯�� (0.198/m)��100%
��������(1)A��SO2����������SO3��������ˮ�ɵ����ᣬ��A��ȷ��B��SO2��Ư���ԣ���Ư��ֽ�ţ���B��ȷ��C��SO2��ǿ�����ԣ�������������ˮ��������C����ΪAB��
(2)����II����As2O3�ܽ���NaOH��Һ����Na3AsO3��AsԪ�صĻ��ϼ�û�з����ı䣬�˷�Ӧ����������ԭ��Ӧ��
(3)����V�ǽ�As2O3������Һͨ�����ˡ�ϴ�ӡ�������ջ��As2O3���壻
(4)������I�б���As2S3����SO2��As2O3��������Ӧ�Ļ�ѧ��Ӧ����ʽΪ2As2S3+9O2![]() 2As2O3+ 6SO2 ��
2As2O3+ 6SO2 ��
������IV��AsO43-��SO2��������������������ԭ��Ӧ����As2O3��SO42-��������Ӧ�����ӷ�Ӧ����ʽΪ 2AsO43-+2SO2+2H+=As2O4��+2SO42-+H2O��
(5)AsO33-ͨ�����ת��ΪAsO43-�Ĺ������������̣�Ӧ��������Χ��������ǿ������Һ�е�⺬AsO33-����Һ�������ĵ缫��ӦʽΪAsO33-+2OH--2e- = AsO43-+ H2O ��
(6)����I2��������Һ����ɫ����ζ��յ�������ǵ����һ��I2����Һ����ʱ����Һ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٷ����仯��
����֪I2+AsO33-=AsO43-+2I-����As2O3~~2AsO33-~~2I2�������εζ����ڶ��ζ�������ƫ��ϴ����һ�κ͵����ε�ƽ���������Ϊ20mL����I2�����ʵ���Ϊ0.02500 mol��L-1��0.02L=0.5��10-3mol������Ʒ��As2O3�����ʵ���Ϊ0.5��10-3mol��2��![]() =1��10-3mol���ֲ�Ʒ��As2O3����������Ϊ
=1��10-3mol���ֲ�Ʒ��As2O3����������Ϊ![]() ��100%= (0.198/m)��100%��
��100%= (0.198/m)��100%��


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ͬ��Ba(OH)2��Һ�У� �ֱ�������ʵ���Ũ����ȵ�H2SO4��NaHSO4��Һ���䵼�������������Һ����仯��������ͼ��ʾ�����з�������ȷ����( )

A��c�㣬����Һ�к�����ͬ����OH��
B��b�㣬��Һ�д������ڵ�������Na����OH��
C���������μ�H2SO4��Һ�ı仯����
D��a��d�����Ӧ����Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش�����������![]()
��1������Ӧ����______________
����Ӧ����________________
��2������������
�� CH3��CH3 �� CH2=CH2 �� CH3CH2C![]() CH
CH
�� CH3C![]() CCH3 �� C2H6 �� CH3CH=CH2
CCH3 �� C2H6 �� CH3CH=CH2
һ����Ϊͬϵ�����_______��һ����Ϊͬ���칹�����_______(�����)
��3��������ë����Ҫ�ɷ��Ǿ۱�ϩ��(��ϩ�棺CH2=CH��CN)����д������Ȳ��HCNΪԭ�ϣ�������Ӧ�ϳɾ۱�ϩ��Ļ�ѧ����ʽ��
��_________________________________
��_________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��ˮ�г�����һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ���˺���������д��������õĴ��������ǻ�ԭ���������÷��Ĺ�������Ϊ��![]()
���е���������ƽ�⣺2 CrO42-(��ɫ)+2H+![]() Cr2O72- (��ɫ)+H2O
Cr2O72- (��ɫ)+H2O
��1�� ��ƽ����ϵ��pH=12������Һ��_______ɫ��
��2�� ��˵����������Ӧ��ƽ��״̬����______________��
a��Cr2O72-��CrO42-��Ũ�Ȳ���
B��2v(Cr2O72-) =v(CrO42-)
C����Һ����ɫ����
��3���������У���ԭlmol Cr2O72-���ӣ���Ҫ_______mol��FeSO47H2O��
��4�����������ɵ�Cr(OH)3����Һ�д������³����ܽ�ƽ�⣺
Cr (OH)3 (s)![]() Cr3+ (aq) +3OH- (aq)
Cr3+ (aq) +3OH- (aq)
�����£�Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH-) = 10-32��Ҫʹ0.01mol/Lc(Cr3+)��ʼ��������Һ��pHӦ����__________����ʹc(Cr3+)����10-5mol/L����Һ��pHӦ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Ӧ������ͨ��ʵ��ⶨ�ģ����л�ѧ��Ӧ���ʵIJ����У��������ݲ����е���( )
ѡ�� | ��ѧ��Ӧ | ��������(��λʱ����) |
A | 2NO2 | ��ɫ��dz |
B | Zn+H2SO4=ZnSO4+H2 | H2��� |
C | CO(g)+H2O(g)=CO2(g)+H2(g) | ѹǿ�仯 |
D | Ca(OH)2+Na2CO3=CaCO3��+2NaOH | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��������������з�Ӧ��3X��Y ![]() 2Z�������ֲ�ͬ����²�õ�����ֵΪ��
2Z�������ֲ�ͬ����²�õ�����ֵΪ��
�� v(X)��1 mol��L-1��s-1�� �� v(Y)��0.5 mol��L-1��s-1�� �� v(Z)��0.5 mol��L-1��s-1��
����������·�Ӧ�����ʴ�С���Ϊ
A. �ۣ��ڣ��� B. �ڣ��٣��� C. �٣��ڣ��� D. �ۣ��٣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��W��X��Y��Z��ԭ�����������������в���Ԫ�������ڱ��е�λ����ͼ��ʾ��һ��WX2�����к���22�����ӣ�Y����������X��Z�ĺ˵����֮�͵ġ��롣����˵����ȷ����
W | X | |
Z |
A. �ǽ����ԣ�W < Z
B. �����ӵİ뾶��X2- < Y2+
C. �е㣺H2X < H2Z
D. WX2�ĵ���ʽΪ��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ɫ��Ӧʵ���õIJ�˿��ÿ����һ����Ʒ������еIJ����ǣ� ��
A. ��ˮϴ��2��3�κ���ʹ��
B. ����ֽ���ɺ�ſ�ʹ��
C. ������ϴ�Ӻ����ھƾ��ƻ��������յ�û����ɫ���ſ�ʹ��
D. ������ϴ�Ӻ�����ˮ��ϴ������ʹ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�ס��ҡ�����X��4����ѧ��ѧ�г���������,��ת����ϵ��ͼ��ʾ��

���X��������
A. ��ΪC��XΪO2
B. ��ΪSO2��XΪNaOH��Һ
C. ��ΪFe��XΪCl2
D. ��Ϊ������Һ��XΪFe
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com