
����Ŀ��ʵ��������500mL 0.2mol/L��NaOH��Һ��
(1)����ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_____________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������__________��____________��

(2)��д���������еĿհף�
���岽�����£�
�ټ�����Ҫ����NaOH���������___________g;
����������ƽ����NaOH���壻
�۽��ƺõ�NaOH��������ձ��У�����������ˮ�ܽ⡢���裬��____________�����£�
�ܽ�NaOH��Һ�ز�����ע��____________�У�
������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲ��ע������ƿ������ζ�����ƿ��ʹ��Һ��Ͼ��ȣ�
������ˮע������ƿ��Һ����̶�����_______cmʱ������____________�μ�����ˮ��Һ���ڿ̶������У�
�߸Ǻ�ƿ�����������µߵ���ҡ�ȣ�
(3)ʹ������ƿǰ������е�һ��������_____________________��
(4)����ȷ���������������Һ���ʵ���Ũ��Ϊ0.192mol/L,ԭ�������___________��
A.ʹ����ֽ����NaOH���壻
B.�ܽ�NaOH����ձ�δ�����ϴ�ӣ�
C.����ƿ��ԭ������������ˮ��
D.����ʱ���õ��������⣻
E.δ��ȴֱ��ת��������ƿ��������ã�
���𰸡�C �ձ� ������ 4.0g ��ȴ 500 mL����ƿ 1��2 ��ͷ�ι� ��© AB
��������
��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ����Ҫ��������Ȼ�������������ȷ����ȱ�ٵ��������ƣ�
��2���ٸ��ݻ�����ʽn = CV�����������m=n M��������������ƽ������ȷ��0.1 g����
������ƿ�������ȣ�
������ƿֻ��һ���̶��ߣ�ֻ���������������Ӧ���������Һ��
���ݶ��ݵIJ�����������
��3����������ƿ��ʹ��ע������������
��4���������������ļ�С����Һ��������ᵼ����ҺŨ�ȵ�ƫС��
��1��CΪ��Һ©������������Һ�����в���ʹ�õ���ƿ�ͷ�Һ©��������500mL 0.2 mol/LNaOH��Һ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬�ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2��3�Σ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������һ�����ʵ���Ũ�ȵ���Һ����Ҫ������Ϊ���ձ�����������
�ʴ�Ϊ��C���ձ�����������
��2�������������m=CVM=0.2mol/L��0.5 L��40 g/mol=4.0 g��
�ʴ�Ϊ��4.0 g��
������ƿ����������NaOH����ˮ���ȣ�����ת��֮ǰ��Ҫ���Ѿ��ܽ�õ�NaOH��ȴ�����£�
�ʴ�Ϊ����ȴ
����һ������Ϊת�ƹ��̣�����ƿֻ��һ���̶��ߣ�ֻ���������������Ӧ���������Һ��
�ʴ�Ϊ��500 mL����ƿ��
������ˮע������ƿ��Һ����̶�����1��2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ���ڿ̶�������,
�ʴ�Ϊ��1��2����ͷ�ιܣ�
��3������ƿ��ƿ�������ƹ�������Ҫҡ�ȣ�Ϊ�˱���©Һ��ʹ������ƿǰ�����Ƿ�©ˮ��
�ʴ�Ϊ����©��
��4�� ʵ����������������ҺŨ��Ϊ0.095mol/L��С��0.1mol/L����ʵ������������������������ļ�С����Һ���������
A. �����������׳��⣬����ֽ�����������ƹ��壬�ᵼ����������������С������ʹ�������ʵ���Ũ��ƫС����A�����������
B. �ܽ����ձ�δ�����ϴ�ӣ���ʹ����������ʧ�����ջᵼ����Һ���������Ƶ����ʵ���Ũ��ƫС����B�����������
C. ��ת�Ʋ����Ժ���Ҫ���ݼ�����ˮ���̶��ߣ��������ƿ��ԭ��������������ˮ����ʵ������Ӱ�죬��C�����������
D. ����ʱ���õ��������⣬��������ȡ�ù��������������ʹ��ʵ����ƫ��D�����������
E.����������δ��ȴֱ��ת��������ƿ���������ʱ��������Һ��ʵ�����ƫС������C = ![]() ���Կ���������ʹ��ʵ����ƫ��E�����������
���Կ���������ʹ��ʵ����ƫ��E�����������
��ѡAB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��Һֻ���ܺ���K����Al3����Fe3����Mg2����Ba2����NH4+��Cl����CO32-��SO42-�е����������ӡ�ijͬѧȡ100 mL����Һ�ֳ����ȷݽ�������ʵ�飺
�ٵ�һ�ݼӹ���������������Һ����ȣ��ռ���0.05 mol�д̼�����ζ�����壬ͬʱ�õ�������Һ�ס�
������Һ����ͨ������Ķ�����̼���壬���ɰ�ɫ���������������ˣ�ϴ�����պõ�1.02 g���塣
�۵ڶ��ݼ��������Ȼ�����Һ�����ɰ�ɫ�����������������ᣬ���������ܽ⣬���˳��������ϴ�ӣ�����õ�11.65 g���塣
��1����ԭ��Һ��һ�����ڵ�������_________________________________��һ�������ڵ�������_____________________������ȷ���Ƿ���ڵ�������___________��
��2��Ϊ��ȷ������ȷ���������Ƿ���ڣ����Բ�ȡ�ķ���Ϊ__________________________________��
��3���ɲ�����ȷ������������Һ�е�Ũ��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ƶ���Ҫ�ɷ�����������������һ�ֳ����IJ���ҩ�ijͬѧΪ�����������Fe2+�Ĵ���,��Ʋ�����ʵ������:
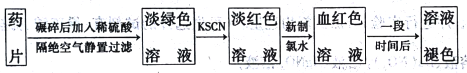
�ش���������:
��1��ʵ��������ҩƬ��Ҫ��������____________��
��2����KSCN��Һ��,��Һ�ʵ���ɫ,��ԭ�������___________������������ˮ��,������Ӧ�����ӷ���ʽΪ_________________��
��3������һ��ʱ���,��Һ����ɫ������ȥ�������Һ��ɫ��ԭ������2�ֲ���:
��� | ���� |
�� | ______ |
�� | _______ |
��4��ҽѧ�Ϸ���ά����C,�ɷ�ֹ�����������ӱ��������ɴ��Ʋ�ά����C����______�ԡ�
��5��������ÿ��Ӧ����14mg���ҵ���,���о���������ʳ����ȫ��ͨ�����ú�FeS04��7H2O��Ƭ����������,��������ÿ������ú�_____mgFeSO4��7H2O��Ƭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��һ���¶��£���1 L 0.1 mol��L��1 CH3COOH��Һ�м���0.1 mol CH3COONa���壬�����ĵ���ƽ����________(��������桱)��Ӧ�����ƶ�����Һ��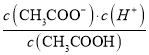 ��ֵ________(���������С�����䡱)��
��ֵ________(���������С�����䡱)��
��2��������ˮ��Һ��Ϊ��ˮ�����д��ڵ���Ҫ��������NH3��H2O��
��֪��a�������£������NH3��H2O�ĵ���ƽ�ⳣ����Ϊ1.74��10��5��
b��CH3COOH��NaHCO3===CH3COONa��CO2����H2O��
��CH3COONH4��Һ��________(��ᡱ��������С�����ͬ)�ԣ�NH4HCO3��Һ��________�ԣ�NH4HCO3��Һ�����ʵ���Ũ������������________(�ѧʽ)��
��3��99 ��ʱ��Kw��1.0��10��12�����¶��²��0.1 mol��L��1 Na2A��Һ��pH��6��
��H2A��ˮ��Һ�еĵ��뷽��ʽΪ______________________________________��
�ڸ��¶��£���0.01 mol��L��1 H2A��Һϡ�͵�20������Һ��pH��________��
�������ȡ�pH��1��������H2A��Һ�ֱ���������Zn��Ӧ������������________��
A������� B��H2A�� C��һ���� D����ȷ��
�ܽ�0.1 mol��L��1H2A��Һ��0.2 mol��L��1��ˮ�������ϣ���ȫ��Ӧ����Һ�и�����Ũ�ȴӴ�С��˳��Ϊ______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A. 32 g O2�����ķ�����ĿΪNA
B. 0.5 mol H2SO4���е�ԭ������ĿΪ3.5NA
C. HNO3��Ħ��������63 g
D. 0.5NA������(Cl2)���ӵ����ʵ�����0.5 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�Ӧ��3S��6KOH![]() 2K2S��K2SO3��3H2O������˵������ȷ���� (����)
2K2S��K2SO3��3H2O������˵������ȷ���� (����)
A. ������������KOH�ǻ�ԭ�� B. ��Ӧ�й�ת��8 mol����
C. ��ԭ��������������������1��2 D. ��������ͻ�ԭ�������������1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼�ǵ���������������������Ԫ��֮һ������Ҫ��ش���������:
��1��̼ԭ�ӵļ۵����Ų�ͼ:_________��������_________�ֲ�ͬ�˶�״̬�ĵ��ӡ�
��2��̼�����γɶ����л��������ͼ��ʾ��һ�����ʺ�һ����वĽṹ�����ַ���������ԭ�Ӷ���һ��ƽ���ϡ�
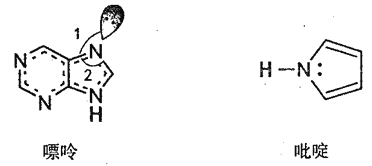
������������Ԫ�صĵ縺���ɴ�С��˳��__________��
�������й��֮��ļнǡ�1�ȡ�2����ԭ��_____________��
����ऽṹ��N ԭ�ӵ��ӻ���ʽ___________��
�ܷ����еĴ����������÷���![]() ��ʾ������m���������γɴ�������ԭ������n���������γɴ������ĵ�����(�籽�����еĴ������ɱ�ʾΪ
��ʾ������m���������γɴ�������ԭ������n���������γɴ������ĵ�����(�籽�����еĴ������ɱ�ʾΪ![]() )�������ʺ�����ж����д�����,���ʸ�����еĴ�������ʾΪ__________��
)�������ʺ�����ж����д�����,���ʸ�����еĴ�������ʾΪ__________��
��3��̼���γ�CO��CO2��H2CO3�ȶ����������
���ڷ�ӦCO ת����CO2 �Ĺ����У�����˵����ȷ����______��
A.ÿ�������й¶Ե��������� B.���Ӽ��Ա仯
C.ԭ�Ӽ�ɼ���ʽ�ı� D.���ӵ��۷е���
���ɱ��ͱ������ֳ����ķ��Ӿ��壬�����еĿռ�������: �ɱ�___����(����>���� ��<������=��)
��H2CO3��H3PO4 ����1�����ǻ�����H3PO4Ϊ��ǿ�ᣬH2CO3Ϊ�����ԭ��______��
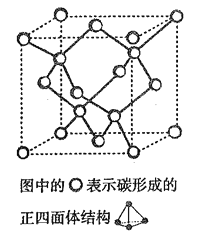
��4����2017 �꣬�����ѧ���Ŷӹ�ͬ�ϳ���̼��һ������ͬ��������: T- ̼��T- ̼�Ľṹ��: ���������ʯ�е�ÿ��̼ԭ����һ����4 ��̼ԭ����ɵ���������ṹ��Ԫȡ�����γ�̼��һ��������ά��������ṹ������ͼ����֪T- ̼��������Ϊa pm������٤������ΪNA����T- ̼���ܶȵı���ʽΪ______g/cm3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�������������
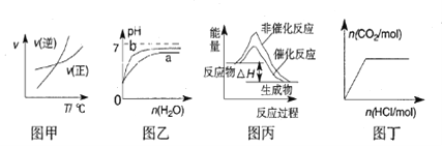
A. ��ͼ�ױ�ʾ�ķ�Ӧ�������¶ȱ仯�Ĺ�ϵ��֪�÷�Ӧ����H>0
B. ͼ�ұ�ʾpH��ͬ�������������ֱ��ˮϡ��PH�ı仯����������a��Ӧ���������
C. ͼ����ʾ�÷�ӦΪ���ȷ�Ӧ���Ҵ����ܸı䷴Ӧ���ʱ�
D. ͼ����ʾ��Na2CO3��Һ����εμ�ϡ���ᣬ����CO2�������������ʵ����Ĺ�ϵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й���Ԫ�������ɵ�������ȷ����
A������Ԫ��ԭ�������ĵ�����ԭ�������������Ǵ�1��8�ظ�����
B������Ԫ��ԭ�������ĵ�����Ԫ��������۴ӣ�1����7�����۴ӣ�7����1�ظ�����
C������Ԫ��ԭ�������ĵ�����ԭ�Ӱ뾶��С����ϡ��������⣩���������Ա仯
D��Ԫ�����ʵ������Ա仯��ָԭ�Ӻ�������Ų���ԭ�Ӱ뾶��Ԫ����Ҫ���ϼ۵������Ա仯
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com