
���� ��1��A��笠�����ˮ�⣬��Һ�����ԣ�B��̼��������Ӵٽ�笠�����ˮ�⣬���Խ��Ȼ����Һ��������C������������ӣ�������笠�����ˮ�⣬��ǿ���ԣ�D��笠�����ˮ�⣬ˮ��̶Ȳ���笠�����Ũ��������Ա��Ȼ����Һ������ǿ��
��2��������������ݼ����������Һ��������Ũ�ȣ����Ksp������Һ��������Ũ�ȣ�
�ڳ�������Һ��ʣ��������Ũ�ȣ�����pH��
��3����NaAˮ��Һ�ʼ��ԣ�˵��A-����ˮ�⣬HA�����ᣬ�����½�0.10molNaA��0.05molHCl����ˮ��������Ӧ��NaA+HCl=NaCl+HA�����ݶ�������õ���Һ�к���0.05molNaA����0.05molHA��0.05molNaCl���õ�pH��7����Һ��˵����Һ�����ԣ�ͬŨ����Һ��HA�������A-���ӵ�ˮ�⣻������Һ�е���غ㣬����غ㣬����Էֱ��������жϣ�
�ڸ��ݵ���غ�ɵ�n��A-��+n��OH-��-n��H+��=c��Na+��-c��Cl-����Ȼ����������ӡ������������ʵ������м��㣻
��� �⣺A��笠�����ˮ�⣬��Һ�����ԣ�B��̼��������Ӵٽ�笠�����ˮ�⣬���Խ��Ȼ����Һ��������C������������ӣ�������笠�����ˮ�⣬��ǿ���ԣ�D��笠�����ˮ�⣬ˮ��̶Ȳ���笠�����Ũ��������Ա��Ȼ����Һ������ǿ��
��pHֵ�ɴ�С��˳���ǣ�B��A��D��C���ʴ�Ϊ��B��A��D��C��
��NH4+����Ũ���ɴ�С��˳���ǣ�D��C��A��B���ʴ�Ϊ��D��C��A��B��
����ͬ�¶��£���ͬ���ʵ���Ũ�ȵ�������ҺpHֵ�ɴ�С��˳���ǣ�B��A��D��C����������Һ��pHֵ��ͬ��������ɵ���Ũ�ȹ�ϵӦ��C��D��A��B���ʴ�Ϊ��C��D��A��B��
��2������50ml0.018mol/L��AgNO3��Һ�м���50ml0.02mol/L�����ᣬ��Ϻ�����Ũ�ȷֱ�Ϊc��Ag+��=$\frac{0.018}{2}$mol/L=0.009 mol/L��c��Cl-��=$\frac{0.02}{2}$mol/L=0.01mol/L��Ag+��Cl-�ǵ����ʵ�����Ӧ�ģ���ʱCl-����������Ũ��c��Cl-��=0.01mol/L-0.009mol/L=0.001mol/L��
Ksp=c��Ag+����C��Cl-��=1.0��10-10����c��Ag+��=$\frac{1.0��1{0}^{-10}}{0.001}$mol/L=1.0��10-7mol/L��
�ʴ�Ϊ��1.0��10-7mol/L��
�ڳ������ɺ�������Ũ��c=$\frac{0.02mol/L}{2}$=0.01mol/L������pH=2��
�ʴ�Ϊ��2��
��3���ٳ����½�0.10mol NaA��0.05mol HCl����ˮ���õ�1L��Һ���õ�����Һ�к���0.05molNaA��0.05molHA��0.05molNaCl����Һ��pH��7��˵����Һ�����ԣ�ͬŨ����Һ��HA�������A-���ӵ�ˮ�⣬c��A-����c��Cl-����c��H+����c��OH-��������Һ������Ũ�ȴ�СΪ��c��Na+����c��A-����c��Cl-����c��H+����c��OH-����
�ʴ�Ϊ��c��Na+����c��A-����c��Cl-����c��H+����c��OH-����
�ڸ��ݻ��Һ�е���غ�ɵã�n��A-��+n��OH-��+c��Cl-��=c��Na+��+n��H+������n��A-��+n��OH-��-n��H+��=c��Na+��-c��Cl-��=0.03mol+0.10nol-0.05mol=0.08mol��
�ʴ�Ϊ��0.08��
���� �����⿼����������ʵĵ��뼰��Ӱ�졢��Һ������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�ע������Ӱ��������ʵĵ���ƽ������أ��ܹ����ݵ���غ㡢�����غ㡢�ε�ˮ���ж���Һ�и�����Ũ�ȴ�С������������ѧ�����Ӧ����ѧ֪ʶ��������


 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ʣ�࣬��Һ�д��ڵĽ�����������Fe2+��Cu2+ | |
| B�� | ��������ʣ�࣬��Һ�д��ڵĽ��������ӿ�����Fe3+��Cu2+ | |
| C�� | ���������������ʣ�࣬��Һ�д��ڵĽ�����������Fe3+��Fe2+��Cu2+ | |
| D�� | ��������ʣ�࣬ʣ�������һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̬�⻯������ȶ��ԣ�Y��Z | |
| B�� | YԪ�ص��������Ӧ��ˮ����һ����ǿ�� | |
| C�� | M��R���γɺ��Ǽ��Լ��Ļ����� | |
| D�� | ����X2Y3����ˮ��Һ����ȡ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��װ�ü����ʵ������ȡ���� | |
| B�� | ��װ���ҳ�ȥ������̼�е������Ȼ��� | |
| C�� | ��װ�ñ�����������̺��Ȼ�����Һ | |
| D�� | ��װ�ö�����̼��������Һ��NaHCO3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ô�ͭ����������ͭ������ | |
| B�� | ���Һ�ijɷֱ��ֲ��� | |
| C�� | �ͽ�����У����������ļ������������������������ | |
| D�� | �����缫��ӦΪ��Cu2++2e-=Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | A | B | C | D |
| ��Ʒ��ǩ |  |  |  |  |
| ���� | ���Լ�Ӧװ���� ������ϸ��ƿ�� | ��ҩƷ������Ƥ��ֱ�ӽӴ� | �����ֽ� | ��ҩƷ��ǩ�ϻ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu ����ϡ HNO3��Cu+2H++NO3-�TCu2++NO2��+H2O | |
| B�� | ��������ˮ��Cl2+H2O�T2H++Cl-+ClO- | |
| C�� | ��CH3COOH�ܽ� CaCO3��CaCO3+2H+�TCa2++H2O+CO2�� | |
| D�� | FeCl3��Һ��Cu��Ӧ��2Fe3++Cu�T2Fe2++Cu2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
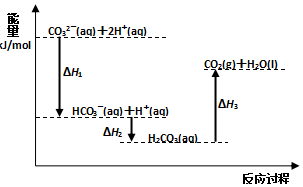
| A�� | ��ӦHCO3-��aq��+H+��aq��=CO2��g��+H2O��l�� Ϊ���ȷ�Ӧ | |
| B�� | CO32-��aq��+2H+��aq��=CO2��g��+H2O��l����H=����H1+��H2+��H3�� | |
| C�� | ��H1����H2��H2����H3 | |
| D�� | H2CO3��aq��=CO2��g��+H2O��l������ʹ�ô��������H3��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
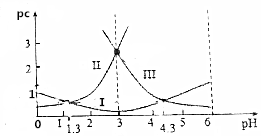 ���ᣨH2C2O4��ˮ��Һ��pc��H2C2O4����pc��HC2O4-����pc��C2O42-��������ҺpH�ı仯������ͼ��ʾ������˵���в���ȷ���ǣ�������
���ᣨH2C2O4��ˮ��Һ��pc��H2C2O4����pc��HC2O4-����pc��C2O42-��������ҺpH�ı仯������ͼ��ʾ������˵���в���ȷ���ǣ�������| A�� | pH=4ʱ��c��HC2O4-����c��C2O42-�� | |
| B�� | c��H2C2O4��+c��HC2O4-��+c��C2O42-��һ������ | |
| C�� | ����ĵ��볣��Ka1=10-1.3 | |
| D�� | $\frac{c��{C}_{2}{{O}_{4}}^{2-}��•c��{H}_{2}{C}_{2}{O}_{4}��}{{c}^{2}��H{C}_{2}{{O}_{4}}^{-}��}$=10-3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com