
����Ŀ��������Ŀ��Ϣ������з���ʽ��
(1)��(Ti)��Ϊ�������������Խ��Խ�������ǵĹ�ע���ؿ��к�������ʯ֮һ�ǽ��ʯ(TiO2)��Ŀǰ���ģ�����ķ����ǣ�
��һ�������ʯ��̿�ۻ�ϣ��ڸ��������£�ͨ��Cl2�Ƶ�TiCl4��һ�ֿ�ȼ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ��____________________________________________��
�ڶ�����������������У��ù�����þ�ڼ�����������TiCl4��Ӧ�Ƶý����ѡ�д���˷�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
(2)��100mL 0.1mol/L��NaOH��Һ����224mLCO2����(��״��)��ǡ����ȫ���ա��ٽ�������Һ����100mL 0.1mol/L����������Һ�С���д��������Һ������������Һ��Ӧ�����ӷ���ʽ��_________________________________________________��
���𰸡� TiO2+2C+2Cl2 ![]() TiCl4+2CO 2Mg+TiCl4
TiCl4+2CO 2Mg+TiCl4![]() 2MgCl2+Ti Ca2++HCO3- + OH- = CaCO3��+ H2O
2MgCl2+Ti Ca2++HCO3- + OH- = CaCO3��+ H2O
��������
��1����һ��:����Ԫ���غ㼰ԭ���غ�ȷ����һ��������,�ٸ��ݷ�Ӧ������P��Ӧ������д����ʽ��
�ڶ���:���ݷ�Ӧ������P��Ӧ������д����ʽ��
��2����100mL0.1mol/L��NaOH����224mLCO2����(��״��)��ǡ����ȫ���ա�n(NaOH)=0.1mol/L��0.1L=0.01mol��n(CO2)=0.224L/22.4L/mol=0.01mol������ǡ�÷�Ӧ����NaHCO3���ٽ�������Һ����100mL0.1mol/L����������Һ��,�����������ʵ���0.1mol/L��0.1 L=0.01mol������̼����������������1:1��ȫ��Ӧ����̼��ơ��������ƺ�ˮ���ݴ�д���÷�Ӧ�����ӷ���ʽ��
(1)��һ��:�ڸ���ʱ,�����ʯTiO2��̿�ۻ�ϲ�ͨ�������Ƶ�TiCl4��һ�ֿ�ȼ����,����Ԫ���غ㼰ԭ���غ�֪,��������CO,���Է�Ӧ����ʽΪ��TiO2+2C+2Cl2 ![]() TiCl4+2CO��������������У��ù�����þ�ڼ�����������TiCl4��Ӧ�Ƶý����ѣ������û���Ӧ����Ӧ�Ļ�ѧ����ʽ��2Mg+TiCl4
TiCl4+2CO��������������У��ù�����þ�ڼ�����������TiCl4��Ӧ�Ƶý����ѣ������û���Ӧ����Ӧ�Ļ�ѧ����ʽ��2Mg+TiCl4![]() 2MgCl2+Ti ��
2MgCl2+Ti ��
����������������ǣ�TiO2+2C+2Cl2 ![]() TiCl4+2CO��2Mg+TiCl4
TiCl4+2CO��2Mg+TiCl4![]() 2MgCl2+Ti ��
2MgCl2+Ti ��
(2)��100mL0.1mol/L��NaOH����224mLCO2����(��״��)��ǡ����ȫ���ա�n(NaOH)=0.1mol/L��0.1L=0.01mol��n(CO2)=0.224L/22.4L/mol=0.01mol,����ǡ�÷�Ӧ����NaHCO3���ٽ�������Һ����100mL0.1mol/L����������Һ��,�����������ʵ���0.1mol/L��0.1 L=0.01mol������̼����������������1:1��ȫ��Ӧ����̼��ơ��������ƺ�ˮ����Ӧ�����ӷ���ʽΪ: Ca2++HCO3-+OH- =CaCO3��+H2O��
������������������Ca2++HCO3-+OH- = CaCO3��+H2O��


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£���Al2O3����C�۵Ļ������ͨ�����������Ƶ���ˮ���AlCl3��ʵ��װ����ͼ��ʾ������˵������ȷ����
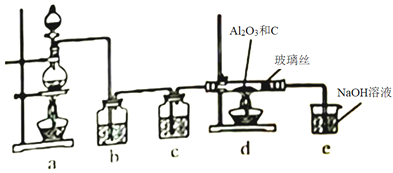
A. c��ʢװ����Ũ����
B. ʵ��ʱӦ�ȵ�ȼd���ƾ��ƣ��ٵ�ȼa���ƾ���
C. �˷�Ӧ�е�β�����������ⶼ��ֱ���ŷŵ�������
D. ��װ�ò����ƣ�����Ӧ�Ľ���һ������d��e֮������һ������װ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ԫ�����ڱ�ǰ�������е����ֳ���Ԫ�����������Ϣ���±���
Ԫ�� | �����Ϣ |
X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е�������ÿ���ܼ��ϵĵ�������� |
Y | ���³�ѹ����Y�����ǵ���ɫ���������ڻ�ɽ�ڸ������� |
Z | Z��Yͬ������Z�ĵ縺�Դ���Y |
W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
��1��Yλ��Ԫ�����ڱ���________���ڵ�________����Y��Z������������Ӧ��ˮ��������Խ�ǿ����________(д��ѧʽ)��
��2��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��___________________________________��
��3��W2Y�ڿ�������������W2O�Ļ�ѧ����ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�Ƽ������߶Ժ���̼���ʵĽ������ĸ�ʴ����Һ����ԵĹ�ϵ�������о�����25��ʱ�ó���ҺpHֵ�����ĸ�ʴӰ���ϵ��ͼ��ʾ,����˵����ȷ���ǣ� ��
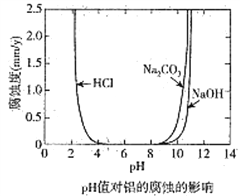
A. ��������Ũ�����еĸ�ʴ���ʴ��������еĸ�ʴ����
B. �����������Ի����в��ױ���ʴ
C. ��������pH=8.5��Na2CO3��Һ�лᷢ���绯ѧ��ʴ��������
D. �����õ��ķ����ڽ������ı����������ܵ������ﱡĤ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D��E��F������������A��E��������������Ԫ������Ҿ���10�����ӣ���������ͼ��ʾ��ת����ϵ��
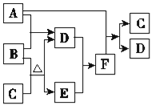
��1��д�����Ŀռ乹�ͣ�A________��C________��
��2���������л�Ϊ�ȵ��������________(д���Ļ�ѧʽ)��
��3��A��E�������к�����λ������________(д���Ļ�ѧʽ)��
��4��E������D����ԭ����_________________��
��5����������F�μӵ�CuSO4��Һ�У���������ɫ����Һ��������ɫ���ʵĻ�ѧʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й��Ŵ��Ĵ���֮һ�����ڻ�ҩ�����ı�ը��ӦΪ2KNO3��3C��S![]() A��N2����3CO2��(����ƽ)
A��N2����3CO2��(����ƽ)
(1)��S�⣬����Ԫ�صĵ縺�ԴӴ�С����Ϊ________��
(2)���������У�A�ľ�������Ϊ________�������Թ��ۼ��ķ��ӵ�����ԭ�ӹ���ӻ�����Ϊ________��
(3)��֪CN����N2�ṹ���ƣ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ӽ���ķ�����ȷ����
A. ij��Һ![]() �а�ɫ������˵��ԭ��Һ����Cl��
�а�ɫ������˵��ԭ��Һ����Cl��
B. ij��Һ![]() �а�ɫ������˵��ԭ��Һ����SO42��
�а�ɫ������˵��ԭ��Һ����SO42��
C. ij��Һ![]() ����ɫ������˵��ԭ��Һ����Cu2+
����ɫ������˵��ԭ��Һ����Cu2+
D. ij��Һ![]() ������ɫ���壬˵��ԭ��Һ����CO32��
������ɫ���壬˵��ԭ��Һ����CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ��ʾһЩ�����е�ijЩ�ṹ�����Ƿֱ���NaCl��CsCl���ɱ������ʯ��ʯī����ṹ�е�ijһ�ֵ�ijһ���֡�
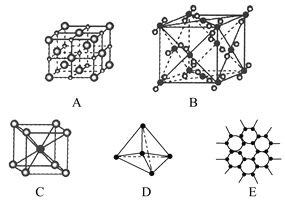
(1)���д������ʯ����(������ĸ����ͬ)________������ÿ��̼ԭ����________��̼ԭ����ӽ��Ҿ�����ȡ����ʯ����________���塣
(2)���д���ʯī����_______������ÿ����������ռ��̼ԭ����ƽ��Ϊ________����
(3)���д���NaCl�������________��ÿ��Na����Χ������ӽ��Ҿ�����ȵ�Na����________����
(4)����CsCl�������________��������________���壬ÿ��Cs����________��Cl�����ڡ�
(5)�����ɱ�����________��������________���壬ÿ��CO2������________��CO2���ӽ��ڡ�
(6)�������������۵��ɸߵ��͵�����˳��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���ڻ�ѧ��������һ�����߱�ʾһ����ѧ��������ͼ��ʾ�����ʽṹ�������߱�ʾ��ѧ������Ӽ�����������________��

��2����֪PH3��NH3�ṹ�������ش��������⣺
��PH3�ĵ���ʽ________________���ṹʽ________________��
�����幹��Ϊ________________��
������ԭ�Ӳ�ȡ________________�ӻ���
��PH3�����еĻ�ѧ��____________(����������������)�����������Ϊ____________(���������������Ǽ�����)���ӡ�
��PH3��NH3�����ȶ��ԣ�____________��ǿ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com