
����Ŀ��(1)��֪�ɽ������Ƶ������ƣ����ö��ַ�����a.4Na��O2===2Na2O��b.4Na��CO2===2Na2O��C��c.2NaNO2��6Na===4Na2O��N2����
�����������ַ����У���õķ�����________(�����)��ԭ����________________��
��������Ӧc��NaNO2��________��������1 mol NaNO2��Ӧʱ������ת�Ƶ���Ŀ��________________________________________________________________________��
(2)���ý����ƺͿ����Ʊ����Ƚϸߵ�Na2O2�������õ�װ�����¡��ش���������(ע��Na2O2������H2O��CO2��Ӧ)��
��װ�â���ʢ�ŵ�ҩƷ��________����������____________________________��

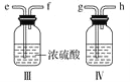
�����涨�����������������ң������ʵ��װ��ʱ�������ӿڵı����ĸ(a��b����)˳��������________��________��________��________��________��________��________��
��װ�â������____________________________________________________��
�ܲ�����ͨ�����ͼ��ȵ�˳��Ϊ________________________________________��
���𰸡�c ��������ֻ��Na2O�ǹ��壬����һ�ֲ���N2������Χ�����е�O2�ž�����ֹNa2O������������Na2O2 ���� 1.806��1024 ����������Һ ���յ���Ŀ����еĶ�����̼ g��h��e��f��a(��b)��b(��a)��c ��ֹ�����е�ˮ�ֺͶ�����̼����װ�â� ��ͨһ��ʱ��Ŀ����ټ���װ�â�
��������
��1������Na2O���ȶ��ױ�������������������𣻸��ݵ�Ԫ�صĻ��ϼ۱仯�����жϺͼ��㣻
��2���ý����ƺͿ����Ʊ����Ƚϸߵ�Na2O2����Ҫ�����е��������Ƽ��ȷ�Ӧ���ɹ������ƣ������е�ˮ�����Ͷ�����̼��Ҫ��ȥ���ѿ���ͨ�������տ����еĶ�����̼���壬��ͨ��װ�â�����ˮ������ͨ��װ�â�����ƺ�������Ӧ�����Ӣ��ֹ�����еĶ�����̼��ˮ��������װ�âò��������Ĺ������ƣ��ݴ˽��
��1����Na2O���ȶ��ױ��������������������������ַ�����õ���c����Ϊ��������ֻ��Na2O�ǹ��壬����һ�ֲ���N2������Χ�����е�O2�ž�����ֹNa2O������������Na2O2��
�ڷ�Ӧ2NaNO2��6Na��4Na2O��N2���е�Ԫ�ػ��ϼ۴�+3�۽��͵�0�ۣ��õ�3�����ӣ���������������������������1 mol NaNO2��Ӧʱ������ת�Ƶ���Ŀ��1mol��3��6.02��1023/mol��1.806��1024��
��2�������ڿ����еĶ�����̼��ˮ����Ҳ��������Ʒ�Ӧ�����Ź������Ƶ��Ʊ�������Ҫ��ȥ�����װ�â���ʢ��NaOH��Һ�������������յ���Ŀ����еĶ�����̼��
�ڰѿ���ͨ�������տ����еĶ�����̼���壬��ͨ��װ�â�����ˮ������ͨ��װ�â�����ƺ�������Ӧ�����Ӣ��ֹ�����еĶ�����̼��ˮ��������װ�â�������ʵ��װ��ʱ����������ȷ����˳��Ϊ��������������ӿڵı����ĸ˳��Ϊ��������g��h��e��f��a����b����b����a����c��
��װ�â���ʢ�ż�ʯ�������ն�����̼��ˮ�������������Ƿ�ֹ�����еĶ�����̼��ˮ��������װ�â�
��ʵ��ʱ��ͨ�������������ٽ��м��ȣ��������ɵĹ������ƺͶ�����̼��ˮ������Ӧ����̼���Ƶ����ʡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
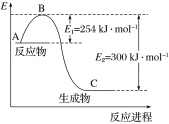
(1)��ͼ��N2(g)��H2(g)��Ӧ����1 mol NH3(g)�����������ı仯ʾ��ͼ����д��N2��H2��Ӧ���Ȼ�ѧ����ʽ��______________��
(2)����֪�������ݣ�
��ѧ�� | H��H | N��N |
����/kJ��mol��1 | 435 | 943 |
�Ը��ݱ��м�ͼ�����ݼ���N��H�ļ��ܣ�________ kJ��mol��1��
(3)��NH3����ԭNOx���������������������Ⱦ����֪��
4NH3(g)��3O2(g)=2N2(g)��6H2O(g) ��H1����a kJ��mol��1��
N2(g)��O2(g)===2NO(g)����H2����b kJ��mol��1��
��1 mol NH3��ԭNO��N2����÷�Ӧ�����еķ�Ӧ����H3��________ kJ��mol��1(�ú�a��b��ʽ�ӱ�ʾ)��
(4)��̼����(��Ҫָ����CO2)�ڽ������������ŷ��о�����Ҫ�����á�ĿǰNH3��(NH4)2CO3�Ѿ���������ҵ��̼����������CO2�ɷ������¿��淴Ӧ��
��Ӧ��2NH3(l)��H2O(l)��CO2(g) ![]() (NH4)2CO3(aq) ��H1
(NH4)2CO3(aq) ��H1
��Ӧ��NH3(l)��H2O(l)��CO2(g) ![]() NH4HCO3(aq)����H2
NH4HCO3(aq)����H2
��Ӧ��(NH4)2CO3(aq)��H2O(l)��CO2(g) ![]() 2NH4HCO3(aq)����H3
2NH4HCO3(aq)����H3
����H3����H1����H2֮��Ĺ�ϵ����H3��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(K2FeO4)Ϊ��Ч��ˮ������ɫ���壬������ˮ������KOH��Һ������ǿ�����ԣ���������Һ����ȫ�����ٲ���O2���ڼ�����Һ�н��ȶ���ijʵ��С���Ʊ�������ز�̽�������ʡ�
(1)�Ʊ�K2FeO4(�г�װ����)
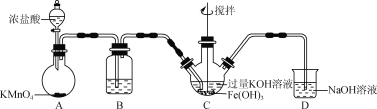
��װ��B�������Լ�Ϊ____��
��C�еõ���ɫ�������Һ��C�з�������Ҫ��ӦΪ________(�û�ѧ����ʽ��ʾ)��
(2) ̽��K2FeO4������
�ٽ�K2FeO4��Һ����MnSO4������H2SO4�Ļ����Һ�У�����Һ��dz��ɫ���������ܷ�֤������������![]() ��
��![]() �����Ե�ǿ����ϵ����˵�����ɣ�____��
�����Ե�ǿ����ϵ����˵�����ɣ�____��
��ȡC����ɫ��Һ������ϡ���ᣬ��������ɫ���壬����Һa�������������к���Cl2��Ϊ֤���Ƿ�K2FeO4������Cl-������Cl2��ijͬѧ��������·�����ȡ����a���μ�KSCN��Һ����������Һ�ʺ�ɫ���÷����������ƣ���Ϊ��Һ����ԭ�������____��___(�����ӷ���ʽ��ʾ)���������ʵ�鷽����֤����K2FeO4������Cl��������Cl2����װ��C�л������ˣ�___��[ʵ���б���ʹ�õ��Լ������ᡢKOH��Һ�����۵⻯����ֽ]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D����Ԫ�أ����ǵ�ԭ����������������С��18��A��B��ͬһ���ڣ�A�ĵ���ʽΪ��![]() ����Bԭ��L��ĵ���������K���3����0.1 mol C�����ܴ������û���2.24 L(��״��)������ͬʱ���ĵ��Ӳ�ṹ�������ԭ����ͬ�ĵ��Ӳ�ṹ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
����Bԭ��L��ĵ���������K���3����0.1 mol C�����ܴ������û���2.24 L(��״��)������ͬʱ���ĵ��Ӳ�ṹ�������ԭ����ͬ�ĵ��Ӳ�ṹ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
(1)д��A��B��C��DԪ�ص����ƣ�A________��B______��C________��D________��
(2)DԪ�������ڱ������ڵ�________����______�塣
(3)�õ���ʽ��ʾA����̬�⻯����γɹ��̣�____________��
(4)A��B�ĵ��ʳ�ַ�Ӧ���ɵĻ�����Ľṹʽ��___________��
(5)B��C�γɵĻ����������ӻ����ﻹ�ǹ��ۻ�������֤����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��ԭ���������ε�����ǰ������Ԫ�أ�AԪ�ص������ϼ��븺���ϼ۵Ĵ�����Ϊ�㣻BԪ��ԭ�ӵļ۵��ӽṹΪnsnnpn��CԪ�ػ�̬ԭ��s�ܼ��ĵ���������p�ܼ��ĵ���������1��DԪ��ԭ�ӵ�M�ܲ�ȫ���������ֻ��һ�����ӡ���ش�
(1)AԪ�ص��ʵĵ���ʽΪ________��BԪ�ص��ʵ�һ�ֿռ���״�ṹ�ľ��壬�۵�>3550 �����õ��ʵľ�����������________________����̬Dԭ�ӹ���__________�ֲ�ͬ�˶�״̬�ĵ��ӡ�
(2)A��C�γɵ�����ӵ�����ԭ���ӻ���ʽ��__________���÷�����D2����H2O��2��1��2����Ƚ���γɵ���������_______���ѧʽ�������������е���������IJ�֮ͬ��Ϊ_____________�����ţ���
������ԭ�ӵļ۲���Ӷ����� ������ԭ�ӵŵ��ӶԵĶ�����
������ԭ�ӵĻ�ѧ������ ��VSEPRģ��
(3)1 mol BC���к��е�������ĿΪ________��д����BC����Ϊ�ȵ�����ķ��Ӻ����Ӹ�һ��________��_________���ѧʽ����
(4)D2+�������ξ�����۵��D2+�������ξ�����۵�ߣ���ԭ����___________________________��
(5)D3C�������õĵ�ѧ��ѧ���ܣ��侧��ľ����ṹ��ͼ��ʾ��D����C3���뾶�ֱ�Ϊa pm��b pm��D����C3�����ǽ��ܽӴ��ĸ���С����C3������λ��Ϊ________��������ܶ�Ϊ_____ g��cm��3��
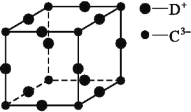
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦA(g) + 3B(g) = 2C(g) + 2D(g)�����ֲ�ͬ����µķ�Ӧ���ʷֱ�Ϊ�� v(A)=0.45 molL1s1 �� v(B)=0.6 molL1s1���� v(C)=0.4 molL1s1���� v(D)=0.45 molL1s1���÷�Ӧ���еĿ���˳��Ϊ�� ��
A.�� > �� = �� > ��B.�� > �� > �� = ��
C.�� > �� = �� > ��D.�� > �� > �� = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�����ʵ���Ũ�ȵ�KOH��Һʱ������Ũ��ƫ�͵�ԭ�������(����)
A. �ó�����������KOH��ʱ�����
B. ����ǰ��������ƿ�м�����������ˮ
C. ����ƿʢ��KOH��Һ��ʹ��ǰδϴ��
D. �ܽ�����ת�Ƶ�����ƿ��Ȼ�����������ˮ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��ͨ������SiO2 ��̼��Ӧ����ȡ�裬д����Ӧ�Ļ�ѧ����ʽ___________________��
��ҵ�ϻ���������þ��ȡ�裬��ӦΪ2Mg+SiO2 = 2MgO+Si��ͬʱ�ᷢ������Ӧ��2Mg + Si = Mg2Si����ͼ�ǽ���Mg��SiO2��Ӧ��ʵ��װ�ã��Իش��������⣺

��1������O2��H2O��g���Ĵ��ڶԸ�ʵ���нϴ�Ӱ�죬ʵ����Ӧͨ������X��Ϊ���������Թ��еĹ���ҩƷ��ѡ��________(�����)��
a��ʯ��ʯ������b��п��������c������
��2��ʵ�鿪ʼʱ��������ͨһ��ʱ��X���壬�ټ��ȷ�Ӧ��������� ___________________________������Ӧ���������߾ƾ��ƣ���Ӧ�ܼ������У���ԭ����______________________��
��3����Ӧ��������ȴ������ʱ������Ӧ��Ļ�����м���ϡ���ᣬ�ɹ۲쵽�����Ļ��ǣ������������ԭ���Ǹ�����Mg2Si������Ѹ�ٷ�Ӧ����SiH4�����飩���壬Ȼ��SiH4��ȼ���û�ѧ����ʽ��ʾ��������Ӧ��________________________��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����KOHΪ����ʵ�ѭ������п�������ε�طŵ�ʱ���ܷ�ӦΪ2Zn+O2=2ZnO������ʱ���ñý�п������Һ�γɵĽ����������ڲ�������Ӧ����Ӧ�����ɵIJ����潬��������غ���������ⲿ�ĵ����У�����ԭ�������������أ�ѭ������п-�������ε�ع�������ͼ��ͼ��ʾ������˵��������ǣ� ��

A.�ŵ�ʱ�����������ӦΪO2+4e-+2H2O=4OH-
B.�ŵ�ʱ��������л���������̼����
C.���ֹͣ����ʱ��п����������Һ����Ӧ
D.���ʱ������������ӦΪZnO+2e-+H2O=Zn+2OH-
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com