
����Ŀ��2001����ΰ��Ļ�ѧ�ҡ�1954��ŵ������ѧ�������������Ļ�ѧ�ṹ��ʦ��20���͵Ŀ�ѧ�ֽܱ���(L.Pauling)���ڵĵ���100���ꡣ1994����λ����л�������Ǵ����İ칫�ң�����������һ��ڰ壬���������ģ�����һ���ṹʽ��ͼ��ʾ������Ϊʲô������ṹʽ�����ܺϳ�������ʲô���ʣ����ö�֪�����DZ����������˵�һ���գ�Ҳ��������Զ������գ�Ҳ���г�һ������ܽ�������ܽ����Σ��������ȶ�����ṹ��һ���˽⡣

(1)���ķ���ʽ��__________________________________________________________��
(2)��������ԭ���Ƿ���ܴ���ͬһ��ƽ���ϣ�________(����ܡ������ܡ�)��
(3)���Ƿ���е�ɣ�________(��ǡ���)��
(4)�÷�����sp�ӻ��ĵ�ԭ����___����sp2�ӻ��ĵ�ԭ����___����sp3�ӻ��ĵ�ԭ����_____����
(5)Ϊʲô�����Ʋ�����ըҩ��______________________________________________��
���𰸡�C6H2O2N10 ���� �� 1 9 0 ���ֽ��ܲ����������ȶ�������N2
��������
��1�����ݽṹ��ʽ��д����ʽ��
��2�����ݱ����Ĺ���ṹ�͵�ԭ�ӵijɼ��ص���ȷ�����л���Ĺ��������
��3������ԭ�ӵijɼ������ȷ�����ԣ�
��4��sp�ӻ���Nԭ�ӱ���Ϊ���ͽṹ��sp2�ӻ�Nԭ�ӱ���Ϊ�����ṹ��sp3�ӻ���Nԭ�ӱ���Ϊ������ṹ��
��5���������д����ĵ�����������Խ��Խ�ȶ����ų�������Խ�ࡣ
��1�����ݽṹ��ʽ��д����ʽΪ��C6H2O2N10���ʴ�Ϊ��C6H2O2N10��
��2��̼ԭ�Ӻ͵�ԭ�Ӿ��൱����3���۵��Ӷԣ����ݼ۲���ӶԻ������ۣ���֪����Ϊƽ�������ͽṹ���ɴ��Ƴ�̼ԭ�Ӻ͵�ԭ�ӹ��ɵĻ�Ϊƽ��ṹ����ԭ����4�Ե��Ӷԣ��������з�ʽΪ������ṹ��������ԭ�����ӵ���ԭ��Ҳ�����ڸ�ƽ���ڣ��뻷��ֱ��������2����ԭ��Ҳ�����ڴ�ƽ���ڣ�������������ԭ�ӿ��ܴ���ͬһ��ƽ�棬�ʴ�Ϊ��������
��3�����е�ԭ�ӳɼ����ﵽ�˱��ͣ����Բ�����ɣ��ʴ�Ϊ����
��4������Nԭ�ӵijɼ��ص�ͽṹ����֪�÷�����sp�ӻ���Nԭ��ֻ��![]() �ṹ���м䵪ԭ��������ĵ�ԭ�Ӷ���sp2�ӻ���û��sp3�ӻ��ģ��ʴ�Ϊ��1��9��0��
�ṹ���м䵪ԭ��������ĵ�ԭ�Ӷ���sp2�ӻ���û��sp3�ӻ��ģ��ʴ�Ϊ��1��9��0��
��5�������ʷֽ������������ȶ�������N2���������仯�Ƕ������������������Խ�ͣ���Ӧ�ų�������Խ�ߣ��ʴ�Ϊ�����ֽ��ܲ����������ȶ�������N2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������ͼ��ʾװ���Ʊ�KClO��Һ������KClO��Һ��KOH��Fe��NO3��3��Һ��Ӧ�Ʊ���Ч��ˮ��K2FeO4��

��֪��Cl2��KOH��Һ��20�����·�Ӧ����KClO���ڽϸ��¶���������KClO3��K2FeO4������ˮ������ŨKOH��Һ����0�桫5���ǿ������Һ�н��ȶ���
�ش��������⣺
��1������a������___________��װ��C������ƿ���ڱ�ˮԡ�е�Ŀ����______________________��
��2��װ��B���յ�������____________��д��ѧʽ����װ��D��������____________________��
��3��װ��C�еõ�����KClO������ƿ�ϵĵ���ȡ�£����μ���KOH��Һ��Fe��NO3��3��Һ������ˮԡ�¶�Ϊ25�棬����1.5 h����Һ��Ϊ�Ϻ�ɫ����K2FeO4������Ӧ�����ӷ���ʽΪ____________________���ټ��뱥��KOH��Һ�������Ϻ�ɫ���壬���ˣ��õ�K2FeO4�ֲ�Ʒ��
��4��K2FeO4�ֲ�Ʒ����Fe��OH��3��KCl�����ʣ����ᴿ����Ϊ����һ������K2FeO4�ֲ�Ʒ�������3 mol��L-1KOH��Һ�У����ˣ���ʢ����Һ���ձ�______________________�����衢���á����ˣ����Ҵ�ϴ�ӹ���2��3�Σ�����������ո������и��
��5���ⶨK2FeO4��Ʒ���ȡ���ȡK2FeO4��Ʒ0.2100 g���ձ��У�����������ǿ�����Ǹ�������Һ����Ӧ���ټ�ϡ���������Һ��ǿ���ԣ����250 mL��Һ��ȡ��25.00 mL������ƿ����0.01000 mol��L-1�ģ�NH4��2Fe��SO4��2��Һ�ζ����յ㣬�ظ�����2�Σ�ƽ�����ı���Һ30.00 mL[��֪��Cr��OH��4-+FeO42-=Fe��OH��3��+CrO42-+OH-��2CrO42-+2H+=Cr2O72-+H2O��Cr2O72-+6Fe2++14H+=6Fe3++3Cr3++7H2O]����K2FeO4��Ʒ�Ĵ���Ϊ_________ %������1λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1869�������ѧ���Ž��з��Ƴ���һ��Ԫ�����ڱ����������γɵ����ڱ��������ڶѧ�ҵļ���Ŭ��������142�꣬Ԫ�����ڱ�������Ԫ��λ���ԵĹ�ϵ����ʾ��Ԫ�ؼ��������ϵ����ͼ��Ԫ�����ڱ���һ���֣��ش��������⡣

��1��Ԫ��Ga��Ԫ�����ڱ��е�λ��Ϊ��___��д�����ں��壩��
��2��Sn���������Ϊ___��Cl������������Ӧˮ����Ļ�ѧʽΪ___��As����̬�⻯��Ϊ___��
��3������Ԫ�������ɣ��ƶϣ�
����Ӱ����Ԫ���⻯�����ȶ�����ߵ���___���ѧʽ����
��H3AsO4��H2SeO4������ǿ����H3AsO4___H2SeO4�����������������=������
���⻯��Ļ�ԭ�ԣ�H2O___H2S�����������������=������
��4������ͼ�зֽ��ߣ����߲��֣�����Ѱ��___������ţ���
A.�����Ĵ��� B.�뵼����� C.�Ͻ���� D.ũҩ
��5����Se2Cl2�����������Լ��������ʽΪ___��
������Se��������In����һ�ֿ�Ӧ����δ�������豸�����Ͱ뵼����ϡ�����˵����ȷ����___������ĸ����
A.ԭ�Ӱ뾶��In��Se
B.In�Ľ����Ա�Seǿ
C.In�Ľ����Ա�Al��
D.�������Ļ�ѧʽΪInSe2
�۹�ҵ�ϳ��ӵ�ұͭ������������ȡ��������������������ͨ��������Cu2Se����Һ�е�HClO����ΪH2SeO3��CuCl2����Ӧ��HClO��Cu2Se�����ʵ���֮��Ϊ___��
��6�������ʵ��Ƚ�C��Si�ķǽ�����ǿ��˳�ɹ�ѡ���ҩƷ�У�CaCO3���塢ϡ���ᡢ���ᡢ����NaHCO3��Һ������Na2CO3��Һ����������Һ����ѧ����������Ҫѡ��
ʵ�鲽�� | ʵ����������� |
���Թ��м���___���ټ���___������������ͨ��___ϴ����ͨ��___�� | ����___�����ۣ��ǽ�����C��Si |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҿ������·�����ȡCl2�����������Ϣ���ش��������⣺
��1����˫���ŷ���ʾ����ת�Ʒ������Ŀ_____����MnO2 +4HCl��Ũ��=== Cl2��+ MnCl2+ 2H2O
��2������Ӧ����0.1mol������������ԭ��������������Ϊ__���ѧʽ���������������ʵ���Ϊ _____��ͬʱת�Ƶ�����Ϊ_____��
��3������2�����ɵ������� 0.2mol H2 ��ȫ��Ӧ�����ɵ������ڱ�״������ռ���Ϊ_____L�����˲�������ˮ���100mL��Һ������Һ�����ʵ���Ũ��Ϊ_______������֪��H2+Cl2![]() 2HCl��
2HCl��
��4����KClO3��6HCl(Ũ)===3Cl2����KCl��3H2O��2KMnO4��16HCl(Ũ)===2KCl��2MnCl2��5Cl2����8H2O
��Ҫ�Ƶ���ͬ�������������٢ڢ�������Ӧ�е���ת�Ƶ���Ŀ֮��Ϊ____��
��5����֪��Ӧ4HCl(g)��O2 ![]() 2Cl2��2H2O(g)���÷�ӦҲ���Ƶ���������MnO2��O2��KMnO4��������������������ǿ��˳��Ϊ_______��
2Cl2��2H2O(g)���÷�ӦҲ���Ƶ���������MnO2��O2��KMnO4��������������������ǿ��˳��Ϊ_______��
��6����������NaOH��Ʒ2.50 g(��Ʒ������Na2CO3��ˮ)������50.0 mL 2.00mol/L�����У���ַ�Ӧ����Һ�����ԣ��кͶ����������ȥ40.0 mL 1.00 mol/L��NaOH��Һ�������кͺ����Һ�����յõ�_____g���塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽��SO2�Ļ�ѧ���ʣ��������ͼϵ��ʵ�顣

��1�����п�ʼʱ��������һ��ʱ����ְ�ɫ���ǣ�д���йص�������Ӧ�Ļ�ѧ����ʽ��___��___��___��
��2�����з�����Ӧ�����Ԫ�صļ�̬Ϊ___��
��3����д���ܢ��з�Ӧ�����ӷ���ʽ��___��___��
��4����ͨ��������ΪCO2����ô�٢ڢۢ����ĸ��������SO2��ͬ������___������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿɽ����ˮ��Դȱ�������⣬�ֿɳ�����ú�����Դ��
(1)��ˮ�д��ڴ������Ȼ��ƣ��Ȼ����еĽ���Ԫ��λ��Ԫ�����ڱ���________�塣
(2)Ŀǰ��������ʵ�õ�����ˮ��������Ҫ����֮һ�����������ǽ���ˮ�����������������ȴ���øߴ��ȵ�ˮ���ɴ˿��ж�������________________(���������仯��������ѧ�仯��)��
(3)��ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ����Ӧʽ��ʳ�Σ�H2O�D��NaOH��H2����Cl2��(δ��ƽ)���÷�Ӧ��ʳ�εĻ�ѧʽ��________________________�����õ������������36.5%��Ũ����1 000 t������������ʳ��________ t��
(4)�����������������һ�������ȼҵ��Ʒ���Ȼ���ѭ������������������������ն�������ķ������÷����������£�

��д���ڢܵĻ�ѧ��Ӧ����ʽ��______��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��������A�е�Ũ����μӵ�ʢ��MnO2����ƿ�У����Ⱥ��������������ͨ��װ��B��C��Ȼ����ͨ�����ȵ�ʯӢ������D�����������ۣ�����ش�
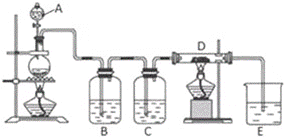
��1������A��������____����ƿ�з�Ӧ�Ļ�ѧ����ʽ��____�������������___������ţ�
�������� �ڻ�ԭ�� ������
��2��װ��B��ʢ��Һ����___������ͨ��װ��B��Ŀ����___��װ��C��ʢ�ŵ�Һ����___��
��3��D�з�Ӧ�Ļ�ѧ����ʽ��____��
��4���ձ�E��ʢ�ŵ�Һ����___��������___����Ӧ�����ӷ���ʽ��___��
��5�����ϱ���D�в������������ʣ�����������������ȴ��������������H2O��g�����ҷ�Ӧ��Ϊ�ռ�D�в����D��E֮�䣬�������ռ�װ���⣬����Ҫ����___װ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��λ������Ϊ����ɫ���壬��ԭ��������С�����A��B��C��D��E����Ԫ����ɣ���ԭ�Ӹ�����Ϊ14��4��5��1��1������C��Dͬ������ԭ������DΪC��2����EԪ�ص���Χ�����Ų�ʽΪ(n��1)dn��6ns1���Իش��������⣺
��1��Ԫ��B��C��D�ĵ�һ�������ɴ�С����˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
��2��DԪ�ػ�̬ԭ�ӵ����������Ų�ͼΪ________��
��3������λ������Ļ�ѧʽΪ________�����������ԭ�ӵ��ӻ���ʽΪ________��
��4��CԪ�ؿ���AԪ���γ����ֳ����Ļ������ԭ�Ӹ����ȷֱ�Ϊ1��1��1��2�����ֻ������������Ȼ��ܣ���������Ҫԭ��Ϊ_______________��
��5��AԪ����BԪ�ؿ��γɷ���ʽΪB2A2��ij������û�����ķ��Ӿ���ƽ��ṹ������ṹʽΪ________�������к���________���Ҽ���________���м���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25�潫Ũ�Ⱦ�Ϊ0.1 mol/L��HA��Һ��BOH��Һ������ֱ�ΪVa��Vb��ϣ�����Va+Vb=100 mL�������ɵ�BA������ˮ����֪Va��Vb����ҺpH��ϵ��ͼ������˵���������

A. ����II��ʾHA��Һ���
B. x�����c(A-)+c(OH-)=c(B+)+c(H+)
C. ����ƽ�ⳣ��K(HA)>K(BOH)
D. ��z����Һ����NaOH��ˮ�ĵ���̶ȼ�С
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com