
����Ŀ����ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ�����ش��������⣺
��1���ñ�������ζ������NaOH��Һʱ���յ�������_______��
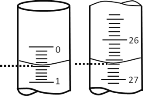
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����ζ�����ʱ�Ķ���Ϊ___________ mL������������Һ�����Ϊ_______mL��
�ζ����� | ����NaOH��Һ�����/mL | 0.1000mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
��3��ijѧ������3��ʵ��ֱ��¼�й��������±���
�����ϱ����ݼ���ɵø�NaOH��Һ�����ʵ���Ũ��Ϊ___mol��L��1��������λ��Ч���֣���
��4�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���_____������ĸ����
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ƿ��ˮϴ�Ӻ��ô���Һ��ϴ
��5������к͵ζ�ԭ��Ҳ�������������͵ĵζ����磺һ�ֲⶨˮ����Br����Ũ�ȵ�ʵ�鲽�����£�
������ƿ�м��봦�����ˮ��25.00mL�����뼸��NH4Fe(SO4)2��Һ��
�ڼ���V1mL c1 mol/L AgNO3��Һ�������������ҡ�ȡ�
����c2mol/L KSCN����Һ���еζ������յ�ʱ���ı���ҺV2mL��
�����ˮ����Br�������ʵ���Ũ��Ϊ_______mol��L��1����֪��Ksp(AgBr��= 7.7��10��13��Ag++ SCN��=AgSCN(��ɫ)�� ��Ksp(AgSCN)= 1��10��12����
��ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L������Һ�����к��ȵIJⶨ��ʵ��װ����ͼ��ʾ��
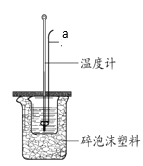
��6������a��������_______��
��7��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬�����ֹ�¶Ȳ��ƽ��ֵΪ4.0�档������Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���ͺ�������Һ�ı�����c��4.18 J/(g����)���������к�����H��______(ȡС�����һλ)��
��8������ʵ�����룭57.3 kJ/mol��ƫ�����ƫ���ԭ�������_____ (����ĸ)��
a��ʵ��װ�ñ��¡�����Ч����
b������Ͳ��ȡNaOH��Һ�����ʱ���ӿ̶��߶���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
���𰸡��������һ�α�Һ����Һ�Ӻ�ɫ�����ɫ��30s�ޱ仯 26.60 26.10 0.2088 B ![]() ���β�������� ��53.5kJ��mol-1 acd
���������� ��53.5kJ��mol-1 acd
��������
��1���ñ�������ζ������NaOH��Һʱ���յ������������ɫ��������ɫ��
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����ζ�����ʱ�Ķ���Ϊ26.60mL������������Һ�����Ϊ26.10mL��
��3���ڶ���ʵ������ʧ�棬��һ������������������ƽ��ֵΪ26.10mL�������ϱ����ݼ���ɵø�NaOH��Һ�����ʵ���Ũ��Ϊ![]() ��
��
��4��A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������ᣬ����Ũ��ƫС���������ƫ���NaOH��Һ��Ũ����ֵƫ�ߣ�A�������⣻
B����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ�������ȡ�����������ֵƫС�����NaOH��Һ��Ũ����ֵƫ�ͣ�B�������⣻
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ����ȡ���������ƫ���NaOH��Һ��Ũ����ֵƫ�ߣ�C�������⣻
D����ƿ��ˮϴ�Ӻ��ô���Һ��ϴ�������������ƫ���NaOH��Һ��Ũ����ֵƫ��D�������⡣
��5����ΪKsp(AgBr)< Ksp(AgSCN)������KSCN������AgBr������Ӧ����ˮ����Br�������ʵ���Ũ��ΪAgNO3����ʼ���ʵ�����ʣ�����ʵ���֮�����ˮ���������
��6������a�������ǻ��β����������
��7��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬�����ֹ�¶Ȳ��ƽ��ֵΪ4.0�榤H��![]() ��
��
��8��a��ʵ��װ�ñ��¡�����Ч�����H��ֵƫС��
b������Ͳ��ȡNaOH��Һ�����ʱ���ӿ̶��߶�����NaOH��ȡ���ƫ���ͷŵ�����ƫ�࣬��H��ֵƫ��
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��ڼ�������е���������ʧ����H��ֵƫС��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ����¶ȼ��ϸ��ŵ�NaOH�����ᷴӦ���ȣ������������ʼ�¶�ƫ�ߣ�����������ֵƫС����H��ֵƫС��
��1���ñ�������ζ������NaOH��Һʱ���յ������ǵ������һ�α�Һ����Һ�Ӻ�ɫ�����ɫ��30s�ޱ仯����Ϊ���������һ�α�Һ����Һ�Ӻ�ɫ�����ɫ��30s�ޱ仯��
��2�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����ζ�����ʱ�Ķ���Ϊ26.60mL������������Һ�����Ϊ26.10mL����Ϊ��26.60��26.10��
��3���ڶ���ʵ������ʧ�棬��һ������������������ƽ��ֵΪ26.10mL�������ϱ����ݼ���ɵø�NaOH��Һ�����ʵ���Ũ��Ϊ![]() =0.2088����Ϊ��0.2088��
=0.2088������0.2088��
��4��A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������ᣬ����Ũ��ƫС���������ƫ���NaOH��Һ��Ũ����ֵƫ�ߣ�A�������⣻
B����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ�������ȡ�����������ֵƫС�����NaOH��Һ��Ũ����ֵƫ�ͣ�B�������⣻
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ����ȡ���������ƫ���NaOH��Һ��Ũ����ֵƫ�ߣ�C�������⣻
D����ƿ��ˮϴ�Ӻ��ô���Һ��ϴ�������������ƫ���NaOH��Һ��Ũ����ֵƫ��D�������⡣��Ϊ��B��
��5����ΪKsp(AgBr)< Ksp(AgSCN)������KSCN������AgBr������Ӧ����ˮ����Br�������ʵ���Ũ��Ϊ![]() mol��L��1����Ϊ��
mol��L��1������![]() ��
��
��6������a�������ǻ��β������������Ϊ�����β����������
��7����H��![]() =��53.5kJ/mol��������53.5kJ/mol��
=��53.5kJ/mol��������53.5kJ/mol��
��8��53.5<57.3������H�IJⶨֵƫ�͡�
a��ʵ��װ�ñ��¡�����Ч�����H��ֵƫС��
b������Ͳ��ȡNaOH��Һ�����ʱ���ӿ̶��߶�����NaOH��ȡ���ƫ���ͷŵ�����ƫ�࣬��H��ֵƫ��
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У��ڼ�������е���������ʧ����H��ֵƫС��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ����¶ȼ��ϸ��ŵ�NaOH�����ᷴӦ���ȣ������������ʼ�¶�ƫ�ߣ�����������ֵƫС����H��ֵƫС��
��acd�������⡣��Ϊ��acd��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴ӦN2(g)+3H2(g) ![]() 2NH3(g) ��H<0,��һ�������´�ƽ�⣬���ı����������仯������������������С������������������ո�
2NH3(g) ��H<0,��һ�������´�ƽ�⣬���ı����������仯������������������С������������������ո�
��1����ѧƽ�ⳣ���ı���ʽΪ _________��
��2�������¶Ⱥ�ѹǿ���䣬����������壬��NH3�����ʵ���_______��
��3�������¶Ⱥ�������䣬����������壬��N2��ת����______��
��4���������ʱ������N2����H2��ת����_______��
����֪CO(g) + H2O(g) ![]() CO2 (g) +H2 (g) 800�� ʱK = 1.0 ���ڴ��¶��µĺ�����ϵ�У�����CO��g����H2O��g������ʼŨ�Ⱦ�Ϊ1mol/L��ʼ��Ӧ��
CO2 (g) +H2 (g) 800�� ʱK = 1.0 ���ڴ��¶��µĺ�����ϵ�У�����CO��g����H2O��g������ʼŨ�Ⱦ�Ϊ1mol/L��ʼ��Ӧ��
��1����COת����Ϊ40%ʱ���÷�Ӧ___����ǡ����ﵽƽ�⣬��ʱ��(��)___��(��) ������ڡ�����С�ڡ����ڡ�����
��2���ﵽƽ��ʱ��H2O��ת����Ϊ_____��
��3����CO����ʼŨ����Ϊ1mol/L��H2O��g������ʼŨ��Ϊ4mol/L��ƽ��ʱH2O��ת����Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(Na3AlF6)�ǹ�ҵ��������Ҫ�ĺ������Ӽ���ʵ�����Է�ʯ(CaF2)��ʯӢ�ʹ���Ϊԭ��ģ�ҵ�Ʊ��������Ƶ��������£�
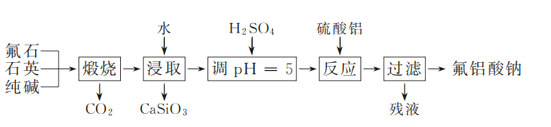
(1) �����ա�ʱ������ҩƷ��Ϻ�Ӧ����________(����������)�м��ȡ�
(2) ͨ�����Ʒ�Һ©���Ļ������Ե�������Һ������ʡ�����ҺpH�ӽ�5ʱ���μ�ϡ����ķ�Һ©���Ļ���Ӧ����ͼ�е�________(�����)��ʾ��

(3) �����Է�ˮ�м���Al2(SO4)3��Na2SO4�����Һ���ɽ���ˮ��F��ת��Ϊ�������Ƴ�����
�� �û����Һ�У�Al2(SO4)3��Na2SO4�����ʵ���֮��Ӧ��________(����ֵ)��
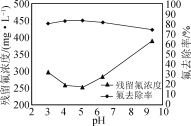
���ڲ��ı���������������£�����NaOH������ҺpH��ʵ������Һ�в�����Ũ�Ⱥͷ�ȥ��������ҺpH�ı仯��ϵ��ͼ��ʾ��pH>5ʱ����Һ�в�����Ũ�������ԭ����________��
(4) ����CaCl2��Ϊ��������ȥF������ʹF��Ũ�Ȳ�����0.95 mg��L��1��c(Ca2��)����Ϊ________mol��L��1��[Ksp(CaF2)��2.7��10��11]
(5) ��ҵ����м(��Ҫ�ɷ�Ϊ����������������������)��������ȡ����������[Al2(SO4)3��18H2O]��
���벹�������ɷ���мΪԭ���Ʊ������������ʵ�鷽����ȡһ��������м�������ձ��У�____________________________________________________________�������������塣[��֪��pH��5ʱ��Al(OH)3������ȫ��pH��8.5ʱ��Al(OH)3������ʼ�ܽ⡣��ʹ�õ��Լ���3 mol��L��1 H2SO4��Һ��2 mol��L��1 NaOH��Һ����ˮ]
��ʵ����������У�Ӧ����ǿ��ͨ�磬ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������G��ɫ��������н�ǿ���������á�G�ɾ���ͼ��ʾ�ϳ�·���Ʊ���

��ش��������⣺
(1) A��B�ķ�Ӧ������________��
(2) E�к��������ŵ�����Ϊ________��________��
(3) C��B��Ϊͬ���칹�壬����С�մ�Ӧ�ų�CO2��������SOCl2����ȡ����Ӧ����D��д��C�Ľṹ��ʽ��________��
(4) д��ͬʱ��������������D��һ��ͬ���칹��Ľṹ��ʽ��________��(�����������칹)
�ٺ�����SH�ṹ��
���������������·���ˮ�ⷴӦ������ˮ�������������ֲ�ͬ������Hԭ�ӡ�����һ��ˮ����������FeCl3��Һ��ɫ��������Br2��CCl4��Һ�����ӳɷ�Ӧ��
(5) д���Լױ����Ҵ�Ϊԭ���Ʊ�![]() �ĺϳ�·������ͼ(���Լ����ã��ϳ�·������ͼʾ�����������)��______________
�ĺϳ�·������ͼ(���Լ����ã��ϳ�·������ͼʾ�����������)��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ����٤������������˵����ȷ���ǣ� ��
A.����£�11.2L�״��к��еĵ�����9NA
B.6.0g��ȩ��HCHO���ʹ���Ļ�����к��е�ԭ������Ϊ0.8NA
C.100mL0.1mol/L��KAl(SO4)2��Һ�к��е�����������С��0.02NA
D.�ֱ���H2O2��KMnO4�Ʊ�����������ת�Ƶĵ�����Ŀ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����÷�ӦNO2��NH3��N2��H2O��δ��ƽ������NO2�ļ���װ����ͼ��ʾ������˵������ȷ���ǣ� ��

A.a�缫��Ӧʽ��![]()
B.���ı�״����![]() ʱ����������NO2�����ʵ���Ϊ
ʱ����������NO2�����ʵ���Ϊ![]()
C.�����ӽ���Ĥ�������ӽ�������װ����������Һ�ļ�����ǿ
D.����װ����NaOH�����ʵ������ϼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����õ�ζ����������һ�ִӻ���������ȡ��ˮ�����ӷ����㾫�ͣ������Ҵ������ѵ��л��ܼ���������ͼ��ʾװ�ô��������ѷۣ��������ᴿ�õ������͡�
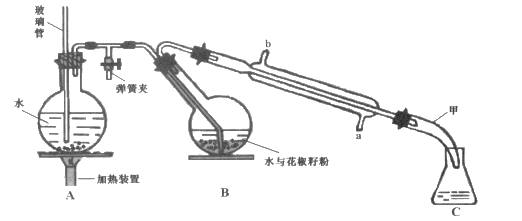
ʵ�鲽�裺
��һ����Aװ���е�Բ����ƿ��װ��![]() �ݻ���ˮ����1~2����ʯ��ͬʱ����B�е�Բ����ƿ�м���20g�����ѷۺ�50mLˮ��
�ݻ���ˮ����1~2����ʯ��ͬʱ����B�е�Բ����ƿ�м���20g�����ѷۺ�50mLˮ��
����������Aװ���е�Բ����ƿ�����д�����������ʱ�رյ��ɼУ���������
�����������Һ�м���ʳ�������ͣ�����15mL������ȡ2�Σ���������ȡ���Ѳ�ϲ�������������ˮNa2SO4����Һ���㵹��������ƿ�У�����û����͡�
(1)װ��A�в����ܵ�������_______��װ��B��Բ����ƿ��б��Ŀ���� ________��
(2)���裨�����У����۲쵽_______����ʱ����ֹͣ�����������ʱ�����в�����˳��Ϊ_______�����ţ���
��ֹͣ���Ȣڴ��ɼТ۹ر�����ˮ
(3)�����Һ�м���ʳ�ε�������__ ��������ˮNa2SO4��������_______��
(4)ʵ���������ϡNaOH��Һ��ϴ�����ܣ���Ӧ�Ļ�ѧ����ʽΪ_________������������![]() ��ʾ��
��ʾ��
(5)Ϊ�ⶨ����������֬�ĺ�����ȡ20.00mL�����������Ҵ��У���80.00mL0.5mol/LNaOH���Ҵ���Һ�����裬��ַ�Ӧ����ˮ���200mL��Һ��ȡ25.00mL�����̪����0.1moI/L������еζ����ζ��յ���������20.00mL����û������к�����֬_______ g/L��
����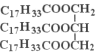 �ƣ�ʽ����884)��
�ƣ�ʽ����884)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������̼��DZ�ڵ�̼��Դ����������Ȼ�Ķ�����̼���أ����Ǹ���¯����β���������������з�����պ���Ũ���������ã������ش�
(1)�ڿռ�վ�г�����CO2(g)+2H2(g)![]() C(s)+2H2O(g)���ٵ��ˮʵ��O2��ѭ�����ã�350��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��8molH2��4molCO2�������Ϸ�Ӧ��
C(s)+2H2O(g)���ٵ��ˮʵ��O2��ѭ�����ã�350��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��8molH2��4molCO2�������Ϸ�Ӧ��
������Ӧ��ʼ��ƽ��ʱ�¶���ͬ(��Ϊ350��)����÷�Ӧ������ѹǿ(p)��ʱ��(t)�ı仯��ͼ��a��ʾ����������Ӧ�ġ�H___________0(�>����<��)������������ͬʱ�������ı�ijһ�����������ѹǿ(p)��ʱ��(t)�ı仯��ͼ������b��ʾ����ı��������___________��
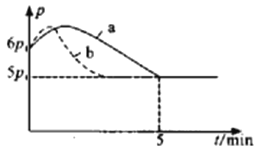
��ͼ�Ƿ�Ӧƽ�ⳣ���Ķ������¶ȵı仯��ϵͼ��m��ֵΪ___________��

(2)CO2�� Cu-ZnO���£�ͬʱ�������·�ӦI��II���ǽ������ЧӦ����Դ��ȱ����Ҫ�ֶΡ�
��.CO2(g)+3H2(g)![]() CH3OH (g)+H2O(g) ��H1<0
CH3OH (g)+H2O(g) ��H1<0
��.CO2(g)+H2(g)![]() CO(g)+ H2O(g) ��H2>0
CO(g)+ H2O(g) ��H2>0
�����¶�Tʱ�����ݻ�������ܱ������У�����һ������CO2��H2����ʼ����ƽ��ʱ�������ڸ��������ʵ�������ѹǿ���±���

����ӦI��II����ƽ��ʱ��p0=1.4p�������n=__________����Ӧ1��ƽ�ⳣ��Kp=____ (kPa)��2��(�ú�p��ʽ�ӱ�ʾ)
(3)Al-CO2�����һ���Ե���������[Al2(CO3)3]Ϊ����ʣ�����ȫ��̼����Pd�����������Ϊ���������Ŀɳ���ء�������ӦΪ��3CO2+4e��=2CO32��+C��������Al�ķ�Ӧ������___________��(�����������)���õ�س��ʱ��Ӧ�Ļ�ѧ����ʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����( )
��֪��2H2(g)��O2(g)=2H2O(l) ��H����571.6 kJmol��1
2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(l) ��H����1452 kJmol��1
H��(aq)��OH��(aq)=H2O(l) ��H����57.3 kJmol��1
A.H2(g)��ȼ����Ϊ571.6 kJmol��1
B.ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų���������
C.H2SO4(aq)��Ba(OH)2(aq)=BaSO4(s)��H2O(l) ��H����57.3 kJmol��1
D.3H2(g)��CO2(g)=CH3OH(l)��H2O(l) ��H����135.9 kJmol��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com