
ij��Һ���п��ܺ������������еļ��֣�K+��NO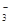 ��SO
��SO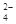 ��NH
��NH ��CO
��CO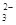 ����������Һ����ˮ�����������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺
����������Һ����ˮ�����������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺
ʵ��1����һ�ݼ����������ռ���ȣ������������ڱ�״����Ϊ672mL��
ʵ��2���ڶ����ȼ������������ᣬ�������ټ���������BaCl2��Һ���ù���2.33g��
��1������Һ��һ�����ڵ������� ��
��2������Һ��һ�������ڵ������� ��
��3������Һ�п��ܴ��ڵ������� ����ó��˽��۵������� ��
��1��NO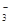 ��SO
��SO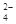 ��NH
��NH ��2��CO
��2��CO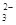
��3��K+ ������֪����Һ�к�NH 0.03mol��SO
0.03mol��SO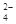 0.01mol���ݵ�����ԭ��������NO
0.01mol���ݵ�����ԭ��������NO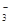 ����NO
����NO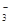 Ϊ0.01mol������K+����NO
Ϊ0.01mol������K+����NO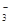 ����0.01mol������K+��
����0.01mol������K+��
���������������1������ʵ��1����һ�ݼ����������ռ���ȣ���NH4++OH-=NH3��+H2O���������״����Ϊ224mL���壬֤������NH4+�������ʵ���Ϊ0.01mol��
ʵ��2���ڶ����ȼ������������ᣬ��������һ��������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤��һ������SO42-�������ʵ���Ϊ��n="m/M" ="2.33g/233g/mol" =0.01mol��������Һ�еĵ���غ㣬��Һ�ʵ����ԣ���һ�����м����ӣ��Ҽ����ӵ�Ũ�ȡ�0.01mol��2?0.01mol�� 0.1L �T0.1mol/L�����Ը���Һ�п϶�����NH4+��S042-��K+��
�ʴ�Ϊ��K+��NH4+��S042-��
��2��ʵ��2���ڶ����ȼ������������ᣬ����������CO32-�������CO32-��CO32-+2H+=H2O+CO2�������ж�����̼����������ʴ�Ϊ��CO32-��
��3�����ݣ�1��֪�������ӵ�Ũ�ȡ�0.01mol��2?0.01mol ��0.1L =0.1mol/L�����K+�����ʵ�������0.01mol����NO3-�����K+�����ʵ�������0.01mol����Ӧ����NO3-��
�ʴ�Ϊ��NO3-��������֪��NH4+���ʵ���Ϊ0.01mol��SO42-���ʵ���Ϊ0.01mol��������Һ�ʵ�����ԭ����Ӧ�ú���K+�����K+�����ʵ�������0.01mol����NO3-�����K+�����ʵ�������0.01mol����Ӧ����NO3-��
���㣺���⿼������Һ�гɷֵļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�������������ɷ�����H1N1���С�����������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��H2SO4��������Na2SO3��Ӧ�Ƶá���д����Ӧ�����ӷ���ʽ_____________________________________________________________________________��
��2��ij��ɫ��Һֻ��������8�������е�ij���֣�Na+��H+��Mg2+��Ag+��Cl-��OH-��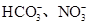 ����֪����Һ����Al2O3��Ӧ����
����֪����Һ����Al2O3��Ӧ����
�ٸ���Һ��Al2O3��Ӧ����Al3+���ɣ���ԭ��Һ��һ������_______��һ�����Ậ�д�����_______��
�ڸ���Һ��Al2O3��Ӧ����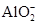 ���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
��д������Һ��Al2O3��Ӧ����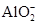 �����ӷ���ʽ____________________________��
�����ӷ���ʽ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����Һ�п��ܴ������е��������±���ʾ��
| ������ | H����K����Al3����NH4+��Mg2�� |
| ������ | Cl����Br����OH����CO32-��AlO2- |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʣ����������ƹ��� ��ͭ˿ ������ �������Ȼ��� �ݶ�����̼���� �ް�ˮ �����Ǿ��塣���������գ�
(1)����״̬�¿ɵ������________�� (2)���ڵ���ʵ���________��
(3)���ڷǵ���ʵ���________�� (4)����״̬�µĵ���ʲ��ܵ������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Na��Ũ��Ϊ0��5mol��L��ij������Һ�У������ܺ����±��е����������ӣ�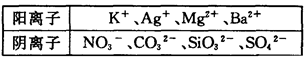
ȡ����Һ100mL��������ʵ�飨��������ڱ�״���²ⶨ����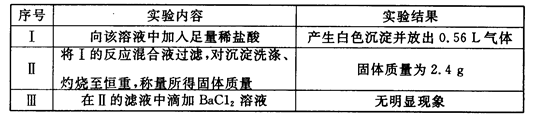
�Իش��������⣺
��1��ʵ�����ȷ��һ�������ڵ���������____________��
��2��ͨ��ʵ���ͱ�Ҫ���㣬��д�±��������ӵ�Ũ�ȣ��ܼ�����ģ���д��������һ�������ڵ������0��������ȷ���Ƿ���ڵ������?����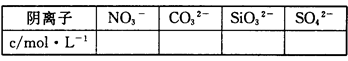
��3���ж�K���Ƿ���ڣ�������������СŨ�ȣ���������˵������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�����ݻ�ѧ����ʽ��д����Ӧ�����ӷ���ʽ��
��AgNO3+KCl=AgCl����KNO3
��CuO+H2SO4=CuSO4��H2O
��2��д��һ����ʵ���������ӷ�Ӧ�Ļ�ѧ����ʽ��
��Fe+Cu2+=Fe2++Cu
��CO32-+2H+=CO2����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I����1����Ba(OH)2��Һ�м���ϡ���ᣬ������������⣺
��1��д����Ӧ�����ӷ���ʽ��________________________________��
��2��������������£����ӷ���ʽ�루1����ͬ����_________________��
A����NaHSO4��Һ����μ���Ba(0H)2��Һ��������
B����NaHSO4��Һ����μ���Ba(0H)2��Һ��SO42��ǡ����ȫ����
C����NaHSO4��Һ����μ���Ba(0H)2��Һ������
��ʵ���ҿ���������غ�Ũ���ᷴӦ��ȡ��������Ӧʽ���£�KClO3��6HCl(Ũ)��KCl��3Cl2����3H2O
��1����˫���ŷ���ʾ������Ӧ�е���ת�Ƶķ������Ŀ��
��2����Ӧ�з���������Ӧ��������____________���ѧʽ��������ԭ��Ԫ����____________________����Ԫ�����ƣ���
��3���������뻹ԭ�������ʵ���֮��Ϊ____________________��
��4������Ӧ�б�����������Ϊ1mol�������ɵ��������Ϊ_______________����״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�������������NaI��KCl��Na2CO3��Na2SO4��CaCl2��Cu(NO3)2�е�һ�ֻ�����ɣ�Ϊ�˼������������ʣ���������ʵ�飺
��ȡ������������ˮ���õ���ɫ����Һ��
��������Һ�еμ��Ȼ�����Һ���а�ɫ�������ɣ�
�۹��ˣ��������м���������ϡ���ᣬ���ֳ���û��ȫ���ܽ�������ɫ��ζ���������ɡ�
������Һ�м������������Ƶ���ˮ���ټ����������ͣ������ã��ϲ�Һ����Ϻ�ɫ��
��1�����жϣ����������п϶����� ��һ��û�� �����ܺ���________________��
��2���Կ��ܺ��е����ʣ���ν���ʵ���Խ�һ�����顣 ��
��3��ʵ����з����Ļ�ѧ��Ӧ���� ��Ӧ���Ӧ���ͣ�����Ҫʵ��������ƽ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ϡ�����ữ�ĺ�6 mol KI��Һ����μ���KBrO3��Һ�����������к���������������KBrO3���ʵ����Ĺ�ϵ��ͼ��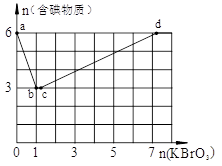
��ش��������⣺
��1��b��ʱ����Ӧ�������ʵĻ�ѧʽΪ ��
��2��b��c�����У�����һ��Ԫ�ط������ϼ۱仯��д���÷�Ӧ�Ļ�ѧ����ʽ���������ת�Ʒ�������Ŀ ��
��3����n(KBrO3)=4molʱ����ϵ�ж�Ӧ�������ʵĻ�ѧʽΪ ��
��4�����������£�Br2��IO3����BrO3����I2��������ǿ������˳��Ϊ ��
��5����ϡ�����ữ��KBrO3��Һ�в��ϵ������KI��Һ���ߵα�����ʵ������еĿ��ܹ۲쵽������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com