
����Ŀ���ߴ�������[Sr(NO3)2]�����������źŵơ���ѧ�����ȡ���ҵ�������Ⱥ�����ơ����ᱵ������,�ᴿ��������:
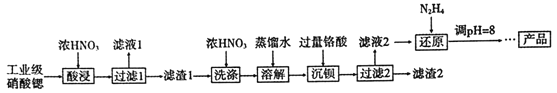
��֪: �١���Һ1������Ҫ������Ca(NO3)2��������1���ijɷ�ΪBa(NO3)2��Sr(NO3)2��������2������Ҫ�ɷ�ΪBaCrO4
�ڸ���(H2CrO4)Ϊ����
(1)����������ܲ��ø��µ�ԭ����_______��
(2)�����ˮϴ,��ŨHNO3ϴ�ӵ��ŵ���_______��
(3)����Һ2���й�����H2CrO4��N2H4��ԭΪCr3+��ͬʱ�ų�����Ⱦ�����壬д����Ӧ�����ӷ���ʽ____��
(4)����Һ�д������³����ܽ�ƽ��:Cr(OH)3(s)![]() Cr3+(aq) +3OH-(aq),�����£�Cr(OH)3���ܶȻ�Ksp=1.0��10-32����c(Cr3+)����1.0��10-5mol/L����ΪCr3+�Ѿ���ȫ�������ֽ���ԭ����Һ��pHֵ����4����ʱCr3+�Ƿ������ȫ? ___( ��ʽ����)��
Cr3+(aq) +3OH-(aq),�����£�Cr(OH)3���ܶȻ�Ksp=1.0��10-32����c(Cr3+)����1.0��10-5mol/L����ΪCr3+�Ѿ���ȫ�������ֽ���ԭ����Һ��pHֵ����4����ʱCr3+�Ƿ������ȫ? ___( ��ʽ����)��
(5)��֪Cr(OH)3����Al(OH)3����ԭ����Һ��pH���ܴ���8��ԭ����_______(������ӷ���ʽ˵������)��
(6)Ϊ�˲ⶨ������2����BaCrO4�ĺ�������������ʵ��:
m g������2��![]() ��Һ
��Һ![]() �ζ��յ�ʱ����VmLNa2S2O3��Һ(��֪:I2+2S2O32-=2I-+S4O62-)
�ζ��յ�ʱ����VmLNa2S2O3��Һ(��֪:I2+2S2O32-=2I-+S4O62-)
���������HI��Һ����̫�࣬�ⶨ�����_____(�ƫ�͡�����ƫ�ߡ�����Ӱ�족)��
�ڡ�����2����BaCrO4(Ħ������ΪMg/mol)����������Ϊ_____(�ô���ʽ��ʾ)��
���𰸡� ����HNO3�ӷ��ͷֽ⣬���ٻ�����Ⱦ ����������(���Ʒ)�ܽ���ʧ 4H2CrO4��3N2H4��12H+��4Cr3+��3N2����16H2O ��pH����4ʱ��c(OH��)= ![]() =1.0��10��10 mol/L��c(Cr3+)=
=1.0��10��10 mol/L��c(Cr3+)= ![]() =1.0��10��2 mol/L��1.0��10��5 mol/L�����Cr3+û�г�����ȫ ����Cr(OH)3�ڼ�����Һ�з���Cr(OH)3��OH����CrO
=1.0��10��2 mol/L��1.0��10��5 mol/L�����Cr3+û�г�����ȫ ����Cr(OH)3�ڼ�����Һ�з���Cr(OH)3��OH����CrO![]() ��2H2O���ܽ� ƫ��
��2H2O���ܽ� ƫ�� ![]()
��������������֪�ٵõ�����ƿ�����Ũ���ᣬ���ᱵ�������Ȳ�����Ũ���ᣬ���Լ���Ũ������˽�������ܽ��ȥ������1�ijɷ��������Ⱥ����ᱵ������1����ϴ�Ӻ��ˮ�ܽ⣬������Ὣ������ת��Ϊ���ᱵ������ȥ������2�ɷ�Ϊ���ᱵ����Һ2��Ҫ�������Ⱥ����ᡢ���ᣬ��N2H4������ת��ΪCr3+����ת��ΪCr(OH)3������ȥ������Ƶ���������
��1�������ǻӷ������������ֽ⣬���ԡ���������ܲ��ø��¡�
��2��������Ӧ������Ũ���������ܣ���ˮ�����ܣ�������Ũ����ϴ����ҪĿ����Ϊ�˽��������ȵ��ܽ�ȣ�������ϴ������������ȵ���ʧ��
��3��H2CrO4��N2H4��ԭΪCr3+��N2H4������Ϊ����Ⱦ��������Ӧ���ǵ�����ǰ��IJ�������������������ڷ�Ӧ���п���������������������Ӧ����ˮ�����Ϸ���ʽΪ��4H2CrO4��3N2H4��12H+��4Cr3+��3N2����16H2O��
��4��pH=4��ʱ���Եõ�c(OH-)=1��10-10mol/L�����Դ�ʱc(Cr3+)=![]() ������Cr3+û�г�����ȫ��
������Cr3+û�г�����ȫ��
��5��Al(OH)3�ڼ�����Һ�лᷴӦת��ΪAlO2-��Cr(OH)3��������Al(OH)3���ƣ�����Ӧ��Ҳ�����ڼ�����Һ���ܽ⣬ת��ΪCrO2-���ӡ����ϣ���ԭ����Һ��pH���ܴ���8��ԭ���ǣ�����Cr(OH)3�ڼ�����Һ�з���Cr(OH)3��OH����CrO2-��2H2O���ܽ⡣
��6������������HI���࣬������HI�ᱻ�����е���������Ϊ���ʵ⣬���Ի����ĸ������������Ƶ���Һ��ʹ�ⶨ���ƫ�ߡ��ڸ��ᱵ�е�+6��CrӦ�ñ������ӻ�ԭΪCr3+,�����ӱ�����Ϊ�ⵥ�ʣ�I2�������ݵ�ʧ�����غ㣬�õ�2BaCrO4��3I2���ٸ�����Ŀ���ķ�Ӧ��I2+2S2O32-=2I-+S4O62-���õ�2BaCrO4��3I2��6S2O32-�����ĵ����������ΪcV��10-3mol������BaCrO4Ϊ![]() ������Ϊ
������Ϊ![]() ��������������Ϊ
��������������Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ײʱ����ȫ�����з�����Ӧ��10NaN3+2KNO3=K2O+5Na2O+16N2��������������Ȼ�ԭ�����1.75 mol���������ж���ȷ���ǣ� ����
A. ����42.0 L N2����״����
B. ��0.250 mol KNO3������
C. ת�Ƶ��ӵ����ʵ���Ϊ1.25 mol
D. ��������Nԭ�ӵ����ʵ���Ϊ1.875 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������G��������Ѫ�ܼ�����ij��ҩ�����Ҫ�м��塣��ϩ��A��������DΪԭ�Ϻϳ�����·����ͼ��ʾ������D����Է�������Ϊ92��C�����к˴Ź�������ֻ��1���塣
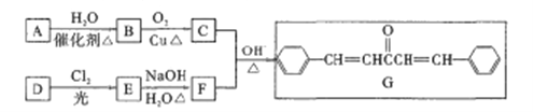
��֪����ͬһ��̼ԭ������2���ǻ�ʱ���Զ���ȥһ��ˮ���ӡ�
��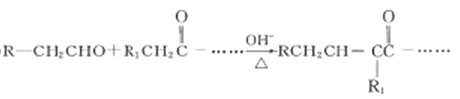
��1��A�ķ���ʽΪ______��B�Ľṹ��ʽΪ______�� G�й�����������________
��2��A��B�ķ�Ӧ������__��F������Ϊ________
��3��д��E��F�Ļ�ѧ����ʽ��____________
��4��E�ж���ͬ���칹�����������ڷ����廯����Ļ���______����д���˴Ź���������3��������ʵĽṹ��ʽ________
��5����ҵ������ȩΪԭ�Ϻϳ�CH3CH2CHClCOOH��·��ͼ������
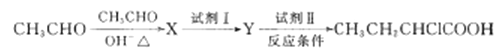
��д��X�Ľṹ��ʽ_______
���Լ�I��_____���Լ���Ӧ�����ֱ�_____�����ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йػ������������ʡ��ǵ���ʵ���ȷ���Ϊ( )
������ | ����� | ����� | �ǵ���� | |
A. | ������ | ˮú�� | ���� | �ɱ� |
B. | ����ˮ | ������Һ | ������ | �������� |
C. | ���� | ������������ | �� | ̼��� |
D. | ˮ�� | ����ʯ��ˮ | �Ȼ�ͭ | ̼���� |
A.A
B.B
C.C
D.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�к��ж��ֳɷ֣�������ж������ʣ�����������ˮ�ֱ�����ͼ�������ʷ����ķ�Ӧ���(a��b��c��d��e���غϲ��ִ������ʼ䷴Ӧ, ����ˮ����)��
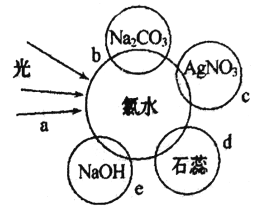
��1����֤����ˮ����Ư���Ե���_____________________(����a����b����c������d��)��
��2��c�����������_____________________________________��
��3��e�����е����ӷ���ʽΪ____________________________________��
��4��ʵ���ұ��汥����ˮ�ķ�����____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���A�ǾۺϷ�Ӧ�����������ϵĵ���,�����Ϊ�ϳɵ����I����������J��ԭ�ϡ���غϳ�·������:
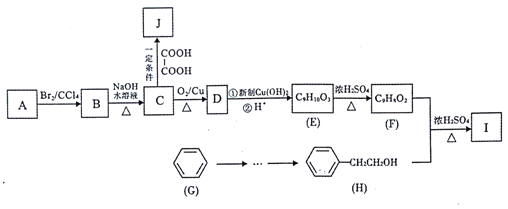
��֪:��A������ͼ�е�����ʺɱ�Ϊ118����˴Ź�����������5��壬�������Ϊ3:2:2:2:1���䱽����ֻ��һ��ȡ������
����������Ϣ�ش���������:
(1)A�ķ���ʽΪ_____��J�Ľṹ��ʽΪ_______��
(2)��B����C�ķ�Ӧ������_______��
(3)E�еĹ���������Ϊ____��
(4)F��H��Ӧ����I�Ļ�ѧ����ʽΪ________��
(5)��������������C ��ͬ���칹����____��(�����������칹)�����к˴Ź�������Ϊ5���Ľṹ��ʽΪ________��
�ٱ�����������ȡ���� �ڱ����ϵ�һ�ȴ���������
�ۼ���FeCl3��Һ����ɫ ����1mol�������м��������Ľ����ƿɲ���1molH2
(6)��֪��![]() (R Ϊ����)��д���ñ�����ϩΪԭ���Ʊ�H�ĺϳ�·��____(�����Լ���ѡ)��
(R Ϊ����)��д���ñ�����ϩΪԭ���Ʊ�H�ĺϳ�·��____(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ��������������ȷ���ǣ� ��
A. ������ԭ����Ϊ0.4NA��CH3OH�����к��еĹ��ۼ���ĿΪ0.5NA
B. a gij���庬������Ϊb��c g�������ڱ�״�������Ϊ![]() L
L
C. ��ⱥ��ʳ��ˮ����������22.4 L����ʱ����·��ͨ���ĵ�����ĿΪ2NA
D. �����£�1.0 L pH��13��Ba(OH)2��Һ�к��е�OH����ĿΪ0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�������Һ�е�����Ũ�ȹ�ϵ��ȷ����
A. NH4C1��Һ����c(Cl-)=c(NH4+)>c(OH-)=c(H+)
B. Na2SO4��Һ����c(Na+) + c(H+)=c(SO42-) + c(OH-)
C. NaHCO3��Һ����c(Na+)> c(HCO3-) >c(CO32-)> c(OH-)
D. Ũ�Ⱦ�Ϊ0.1mol/LCH3COOH ��CH3COONa��Һ�������ϣ�c(CH3COO-) + c(CH3COOH)=0.1mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������ǹ�ҵ����Ҫ�Ļ���ԭ�ϣ�Ҳ��ʵ�����ﳣ�����Լ���
�ⶨ�к��ȣ�
д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ__________________________
���к�����ֵΪ57.3kJ/mol����
��2��ȡ50mL 0.5mol/L HCl��Һ��50mL0.55mol/L NaOH��Һ���вⶨ����ʵ����ֵС��57.3kJ/mol��ԭ������_______________������ţ���
A�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������¶�
B����ȡ��������ʱ���Ӷ���
C���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D��ʵ��װ�ñ��¡�����Ч����
������к͵ζ���
���ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000 mol��L-1 HCl����Һ�����к͵ζ�(�÷�̪��ָʾ��)����ش��������⣺
��1������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ1.10 mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ_________________��

��2����ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol��L-1HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.11 |
2 | 25.00 | 1.56 | 31.30 |
3 | 25.00 | 0.22 | 26.31 |
ѡȡ�����������ݣ����������NaOH��Һ�����ʵ���Ũ��Ϊ________________(С���������λ)��
��3��������Щ������ʹ�ⶨ���ƫ��___________(�����)��
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
�ζ��յ���ж�__________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com