
ΓΨΧβΡΩΓΩ25Γφ ±Θ§≤ΩΖ÷Έο÷ ΒΡΒγάκΤΫΚβ≥Θ ΐ»γ±μΥυ ΨΘΚ
Μ·―ß Ϋ |
|
| HClO |
|
|
ΒγάκΤΫΚβ≥Θ ΐ |
|
|
|
|
|
Θ®1Θ©25Γφ ±Θ§Β»≈®Ε»ΒΡ![]() »ή“ΚΓΔ
»ή“ΚΓΔ![]() »ή“ΚΓΔ
»ή“ΚΓΔ![]() »ή“ΚΘ§3÷÷»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ________ΓΘ
»ή“ΚΘ§3÷÷»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ________ΓΘ
Θ®2Θ©ΙΛ“Β…œΩ…”ΟΑ±Υ°≥ΐ»ΞΈ≤Τχ![]() ΓΘΫΪ
ΓΘΫΪ![]() Ά®»κΑ±Υ°÷–Θ§Β±
Ά®»κΑ±Υ°÷–Θ§Β±![]() ΫΒ÷Ν
ΫΒ÷Ν![]() _____ΓΘ
_____ΓΘ
Θ®3Θ©≥ΘΈ¬œ¬Θ§”Ο![]() »ή“ΚΒΈΕ®
»ή“ΚΒΈΕ®![]() »ή“ΚΥυΒΟΒΈΕ®«ζœΏ»γΆΦΘΚ
»ή“ΚΥυΒΟΒΈΕ®«ζœΏ»γΆΦΘΚ
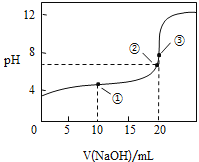
ΔΌ‘Ύ’ϊΗω Β―ιΙΐ≥Χ÷–Θ§≤Μ–η“ΣΒΡ“«ΤςΜρ”ΟΤΖ « ______Θ®Χν–ρΚ≈Θ©Θ°
![]() »ίΝΩΤΩ b ΉΕ–ΈΤΩc ΒΈΕ®ΙήΦ–d ¬©ΕΖe ≤ΘΝßΑτf ΒΈΕ®Ιή
»ίΝΩΤΩ b ΉΕ–ΈΤΩc ΒΈΕ®ΙήΦ–d ¬©ΕΖe ≤ΘΝßΑτf ΒΈΕ®Ιή
ΔΎΒΫ¥οΒΈΕ®÷’ΒψΒΡ±ξ÷Ψ « _____________ Θ°
Δέœ¬Ν–≤ΌΉςΜαΒΦ÷¬≤βΕ®ΫαΙϊΤΪΗΏΒΡ « ______ Θ°
A Φν ΫΒΈΕ®Ιή‘ΎΉΑ“Κ«ΑΈ¥”Ο±ξΉΦNaOH»ή“Κ»σœ¥
B ΒΈΕ®Ιΐ≥Χ÷–Θ§ΉΕ–ΈΤΩ“ΓΒ¥ΒΟΧΪΨγΝ“Θ§ΉΕ–ΈΤΩΡΎ”–“ΚΒΈΫΠ≥ω
C Φν ΫΒΈΕ®ΙήΦβΉλ≤ΩΖ÷‘ΎΒΈΕ®«ΑΟΜ”–Τχ≈ίΘ§ΒΈΕ®÷’Βψ ±ΖΔœ÷Τχ≈ί
D ¥οΒΫΒΈΕ®÷’Βψ ±Θ§―ω ”ΕΝ ΐ
Δή»γΆΦΒψΔΌΥυ Ψ»ή“Κ÷–![]() __________
__________![]() ΧνΓΑΘΨΓ±ΓΑΘΦΓ±ΜρΓΑ=Γ±Θ§œ¬Ά§Θ§ΒψΔΎΥυ Ψ»ή“Κ÷–ΘΚ
ΧνΓΑΘΨΓ±ΓΑΘΦΓ±ΜρΓΑ=Γ±Θ§œ¬Ά§Θ§ΒψΔΎΥυ Ψ»ή“Κ÷–ΘΚ![]() ________
________![]() Θ§ΒψΔέΥυ Ψ»ή“Κ÷–Υυ”–άκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣΘΚ_________ΓΘ
Θ§ΒψΔέΥυ Ψ»ή“Κ÷–Υυ”–άκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣΘΚ_________ΓΘ
ΓΨ¥πΑΗΓΩNa2CO3»ή“ΚΘΨNa2SO3»ή“ΚΘΨCH3COONa»ή“Κ 0.62 ade Φ”»κΉνΚσ“ΜΒΈ«β―θΜ·ΡΤΘ§»ή“Κ±δΈΣΈΔΚλ…ΪΘ§«“30sΡΎ≤ΜΆ …Ϊ AD ΘΨ ΘΦ ![]()
ΓΨΫβΈωΓΩ
(1)ΥαΒΡΒγάκΤΫΚβ≥Θ ΐ‘Ϋ¥σΘ§ΤδΕ‘”ΠΒΡΥαΗυάκΉ”Υ°Ϋβ≥ΧΕ»‘Ϋ–ΓΘ§Β»≈®Ε»ΒΡΡΤ―Έ»ή“ΚΒΡpH‘Ϋ–ΓΘΜ
(2) ![]() =
= ![]() ΓΝ
ΓΝ![]() =
=![]() Ζ÷ΈωΦΤΥψΘΜ
Ζ÷ΈωΦΤΥψΘΜ
(3)ΔΌΥαΦν÷–ΚΆΒΈΕ® Β―ι÷––η“ΣΒΡ“«ΤςΈΣΥα ΫΒΈΕ®ΙήΓΔΦν ΫΒΈΕ®ΙήΓΔΒΈΕ®ΙήΦ–ΓΔΧζΦήΧ®ΓΔΫΚΆΖΒΈΙήΓΔ…’±≠ΓΔΉΕ–ΈΤΩΒ»“«ΤςΘΜΔΎNaOH»ή“Κ…‘ΙΐΝΩ ±Θ§Ζ”ΧΣ±δΈΣ«≥Κλ…ΪΘΜΔέΗυΨίc(¥ΐ≤β)= ![]() Ζ÷Έω≤ΜΒ±≤ΌΉςΕ‘V(±ξΉΦ)ΒΡ”ΑœλΘ§“‘¥Υ≈–Εœ≈®Ε»ΒΡΈσ≤νΘΜΔή”…ΆΦΩ…÷ΣΘ§ΒψΔΌΥυ Ψ»ή“ΚΈΣΒ»≈®Ε»ΒΡCH3COOHΚΆCH3COONaΒΡΜλΚœ»ή“ΚΘ§»ή“Κ≥ Υα–‘Θ§‘ρc(H+)ΘΨc(OH-)Θ§ΫαΚœ÷ Ή”ΙΊœΒΈΣc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)Ζ÷ΈωΫβ¥πΘΜΒψΔΎΥυ Ψ»ή“Κ≥ ÷––‘Θ§‘ρc(H+)=c(OH-)Θ§ΗυΨίΒγΚ…ΙΊœΒΖ÷ΈωΫβ¥πΘΜΒψΔέ«ΓΚΟ…ζ≥…¥ΉΥαΡΤΘ§ΗυΨί―ΈάύΥ°ΫβΧΊΒψΖ÷Έω≈–ΕœΓΘ
Ζ÷Έω≤ΜΒ±≤ΌΉςΕ‘V(±ξΉΦ)ΒΡ”ΑœλΘ§“‘¥Υ≈–Εœ≈®Ε»ΒΡΈσ≤νΘΜΔή”…ΆΦΩ…÷ΣΘ§ΒψΔΌΥυ Ψ»ή“ΚΈΣΒ»≈®Ε»ΒΡCH3COOHΚΆCH3COONaΒΡΜλΚœ»ή“ΚΘ§»ή“Κ≥ Υα–‘Θ§‘ρc(H+)ΘΨc(OH-)Θ§ΫαΚœ÷ Ή”ΙΊœΒΈΣc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)Ζ÷ΈωΫβ¥πΘΜΒψΔΎΥυ Ψ»ή“Κ≥ ÷––‘Θ§‘ρc(H+)=c(OH-)Θ§ΗυΨίΒγΚ…ΙΊœΒΖ÷ΈωΫβ¥πΘΜΒψΔέ«ΓΚΟ…ζ≥…¥ΉΥαΡΤΘ§ΗυΨί―ΈάύΥ°ΫβΧΊΒψΖ÷Έω≈–ΕœΓΘ
(1)ΥαΒΡΒγάκΤΫΚβ≥Θ ΐ‘Ϋ¥σΘ§ΤδΕ‘”ΠΒΡΥαΗυάκΉ”Υ°Ϋβ≥ΧΕ»‘Ϋ–ΓΘ§Β»≈®Ε»ΒΡΡΤ―Έ»ή“ΚΒΡpH‘Ϋ–ΓΘ§ΒγάκΤΫΚβ≥Θ ΐΘΚCH3COOHΘΨHSO3-ΘΨHCO3-Θ§‘ρΥ°Ϋβ≥ΧΕ»ΘΚCH3COO-ΘΦSO32-ΘΦCO32-Θ§Υ°Ϋβ≥ΧΕ»‘Ϋ¥σΤδœύΆ§≈®Ε»ΒΡΡΤ―Έ»ή“ΚΒΡpH‘Ϋ¥σΘ§‘ρ3÷÷»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣΘΚNa2CO3»ή“ΚΘΨNa2SO3»ή“ΚΘΨCH3COONa»ή“ΚΘ§Ι ¥πΑΗΈΣΘΚNa2CO3»ή“ΚΘΨNa2SO3»ή“ΚΘΨCH3COONa»ή“ΚΘΜ
(2)![]() =
= ![]() ΓΝ
ΓΝ![]() =
=![]() =
=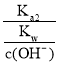 =
=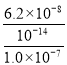 =0.62Θ§Ι ¥πΑΗΈΣΘΚ0.62ΘΜ
=0.62Θ§Ι ¥πΑΗΈΣΘΚ0.62ΘΜ
(3)ΔΌΥαΦν÷–ΚΆΒΈΕ® Β―ι≤ΌΉςΘ§–η“ΣΥα ΫΒΈΕ®ΙήΓΔΦν ΫΒΈΕ®ΙήΓΔΒΈΕ®ΙήΦ–ΓΔΧζΦήΧ®ΓΔ…’±≠ΓΔΉΕ–ΈΤΩΒ»“«ΤςΘ§≤Μ–η“Σ»ίΝΩΤΩΓΔ¬©ΕΖΓΔ≤ΘΝßΑτΘ§Ι ¥πΑΗΈΣΘΚadeΘΜ
ΔΎNaOH»ή“Κ…‘ΙΐΝΩ ±Θ§Ζ”ΧΣ±δΈΣ«≥Κλ…ΪΘ§Υυ“‘NaOH»ή“ΚΒΈΕ®CH3COOH¥οΒΫΒΈΕ®÷’Βψ ±Θ§Φ”»κΉνΚσ“ΜΒΈNaOH»ή“ΚΘ§Ζ”ΧΣ±δΈΣ«≥Κλ…ΪΘ§≤Δ«“30sΡΎ≤ΜΆ …ΪΘ§
Ι ¥πΑΗΈΣΘΚΦ”»κΉνΚσ“ΜΒΈ«β―θΜ·ΡΤΘ§»ή“Κ±δΈΣ«≥Κλ…ΪΘ§«“30sΡΎ≤ΜΆ …ΪΘΜ
ΔέAΘ°Φν ΫΒΈΕ®Ιή‘ΎΉΑ“Κ«ΑΈ¥”Ο±ξΉΦNaOH»ή“Κ»σœ¥Θ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΤΪΗΏΘ§ΗυΨίc(¥ΐ≤β)= ![]() Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΗΏΘ§Ι A―ΓΘΜBΘ°ΒΈΕ®Ιΐ≥Χ÷–Θ§ΉΕ–ΈΤΩ“ΓΒ¥ΒΟΧΪΨγΝ“Θ§ΉΕ–ΈΤΩΡΎ”–“ΚΒΈΫΠ≥ωΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΤΪΒΆΘ§ΗυΨίc(¥ΐ≤β)=
Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΗΏΘ§Ι A―ΓΘΜBΘ°ΒΈΕ®Ιΐ≥Χ÷–Θ§ΉΕ–ΈΤΩ“ΓΒ¥ΒΟΧΪΨγΝ“Θ§ΉΕ–ΈΤΩΡΎ”–“ΚΒΈΫΠ≥ωΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΤΪΒΆΘ§ΗυΨίc(¥ΐ≤β)= ![]() Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΒΆΘ§Ι B≤Μ―ΓΘΜCΘ°Φν ΫΒΈΕ®ΙήΦβΉλ≤ΩΖ÷‘ΎΒΈΕ®«ΑΟΜ”–Τχ≈ίΘ§ΒΈΕ®÷’Βψ ±ΖΔœ÷Τχ≈ίΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪΒΆΘ§ΗυΨίc(¥ΐ≤β)=
Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΒΆΘ§Ι B≤Μ―ΓΘΜCΘ°Φν ΫΒΈΕ®ΙήΦβΉλ≤ΩΖ÷‘ΎΒΈΕ®«ΑΟΜ”–Τχ≈ίΘ§ΒΈΕ®÷’Βψ ±ΖΔœ÷Τχ≈ίΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪΒΆΘ§ΗυΨίc(¥ΐ≤β)= ![]() Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΒΆΘ§Ι C≤Μ―ΓΘΜDΘ°¥οΒΫΒΈΕ®÷’Βψ ±Θ§―ω ”ΕΝ ΐΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪΗΏΘ§ΗυΨίc(¥ΐ≤β)=
Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΒΆΘ§Ι C≤Μ―ΓΘΜDΘ°¥οΒΫΒΈΕ®÷’Βψ ±Θ§―ω ”ΕΝ ΐΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪΗΏΘ§ΗυΨίc(¥ΐ≤β)= ![]() Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΗΏΘ§Ι D―ΓΘΜΙ ¥πΑΗΈΣΘΚADΘΜ
Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΗΏΘ§Ι D―ΓΘΜΙ ¥πΑΗΈΣΘΚADΘΜ
Δή”…ΆΦΩ…÷ΣΘ§ΒψΔΌΥυ Ψ»ή“ΚΈΣΒ»≈®Ε»ΒΡCH3COOHΚΆCH3COONaΒΡΜλΚœ»ή“ΚΘ§»ή“Κ≥ Υα–‘Θ§‘ρc(H+)ΘΨc(OH-)Θ§¥Υ ±÷ Ή”ΙΊœΒΈΣc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)Θ§Φ¥c( CH3COO-)+c(OH-)=c(CH3COOH)+c(H+)+[(H+)-c(OH-)]ΘΨc(CH3COOH)+c(H+)ΘΜΒψΔΎΥυ Ψ»ή“Κ≥ ÷––‘Θ§‘ρc(H+)=c(OH-)Θ§ΒγΚ…ΙΊœΒΈΣc( CH3COO-)+c(OH-)=c(Na+)+c(H+)Θ§Φ¥c(Na+)=c( CH3COO-)Θ§ΒΪ»ή“Κ÷–ΜΙ”–ΈΔΝΩCH3COOHΘ§Υυ“‘c(Na+)ΘΦc( CH3COO-)+c(CH3COOH)ΘΜΒψΔέ«ΓΚΟ…ζ≥…¥ΉΥαΡΤΘ§ΗυΨί―ΈάύΥ°ΫβΧΊΒψΩ…÷ΣΘΚ![]() ΘΜΙ ¥πΑΗΈΣΘΚΘΨΘΜΘΦΘΜ
ΘΜΙ ¥πΑΗΈΣΘΚΘΨΘΜΘΦΘΜ![]() ΓΘ
ΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ…ηNAΈΣΑΔΖπΦ”Β¬¬ό≥Θ ΐΘ§œ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «( )
A.±ξΉΦΉ¥Ωωœ¬Θ§22.4 LΚΛΤχ÷–Κ§2NAΗωΚΛ‘≠Ή”
B.18gΥ°÷–Κ§”–ΒΡΒγΉ” ΐΈΣ10NA
C.1mol Cl2”κΫπ τNaΆξ»ΪΖ¥”ΠΘ§Ω…“‘ΒΟΒΫ2NAΗωΒγΉ”
D.‘Ύ1L2mol/LΒΡœθΥαΟΨ»ή“Κ÷–Κ§”–ΒΡœθΥαΗυάκΉ” ΐΈΣ4NA
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ÷ςΉε‘ΣΥΊWΓΔXΓΔYΓΔZΒΡ‘≠Ή”–ρ ΐ“ά¥Έ‘ωΦ”Θ§«“Ψυ≤Μ¥σ”Ύ20ΓΘWΓΔXΓΔZΉε–ρ ΐ÷°ΚΆΈΣ10ΘΜYΒΡ‘≠Ή”ΑκΨΕ‘ΎΆ§÷ήΤΎ÷ςΉε‘ΣΥΊ÷–Ήν–ΓΘΜW”κZ–Έ≥…ΒΡΜ·ΚœΈο≥ΘΈ¬œ¬Ω…”κ±ΞΚΆNaCl»ή“ΚΖ¥”ΠΘ§≤ζ…ζΒΡΤχΧε≥Θ”Ο”Ύ«–ΗνΚΆΚΗΫ”Ϋπ τΓΘœ¬Ν–ΥΒΖ®¥μΈσΒΡ «

A. XΒΡΒΞ÷ «»ΥάύΫΪΧΪ―τΡήΉΣΜ·ΈΣΒγΡήΒΡ≥Θ”Ο≤ΡΝœ
B. Y‘ΣΥΊΩ…“‘–Έ≥…Εύ÷÷Κ§―θΥα
C. X‘ΣΥΊ‘ΎΉ‘»ΜΫγ÷–÷Μ”–Μ·ΚœΧ§ΟΜ”–”ΈάκΧ§
D. W”κZ–Έ≥…ΒΡΜ·ΚœΈο”κ±ΞΚΆNaCl»ή“ΚΒΡΖ¥”Π“Σ―Γ”Ο»γ…œΆΦΉΑ÷Ο
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ‘ΣΥΊΗθ![]() ‘Ύ»ή“Κ÷–÷ς“Σ“‘
‘Ύ»ή“Κ÷–÷ς“Σ“‘![]() άΕΉœ…Ϊ
άΕΉœ…Ϊ![]() ΓΔ
ΓΔ![]() ¬Χ…Ϊ
¬Χ…Ϊ![]() ΓΔ
ΓΔ![]() ≥»Κλ…Ϊ
≥»Κλ…Ϊ![]() ΓΔ
ΓΔ![]() ΜΤ…Ϊ
ΜΤ…Ϊ![]() Β»–Έ Ϋ¥φ‘ΎΘ§
Β»–Έ Ϋ¥φ‘ΎΘ§![]() ΈΣΡ―»ή”ΎΥ°ΒΡΜ“άΕ…ΪΙΧΧεΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΈΣΡ―»ή”ΎΥ°ΒΡΜ“άΕ…ΪΙΧΧεΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©![]() ”κ
”κ![]() ΒΡΜ·―ß–‘÷ œύΥΤΘ§‘Ύ
ΒΡΜ·―ß–‘÷ œύΥΤΘ§‘Ύ![]() »ή“Κ÷–÷πΒΈΦ”»κNaOH»ή“Κ÷±÷ΝΙΐΝΩΘ§Ω…≤λΒΫΒΡœ÷œσ « ______________________________________________ Θ°
»ή“Κ÷–÷πΒΈΦ”»κNaOH»ή“Κ÷±÷ΝΙΐΝΩΘ§Ω…≤λΒΫΒΡœ÷œσ « ______________________________________________ Θ°
Θ®2Θ©![]() ΚΆ
ΚΆ![]() ‘Ύ»ή“Κ÷–Ω…œύΜΞΉΣΜ·
‘Ύ»ή“Κ÷–Ω…œύΜΞΉΣΜ·![]() “Έ¬œ¬Θ§≥θ Φ≈®Ε»ΈΣ
“Έ¬œ¬Θ§≥θ Φ≈®Ε»ΈΣ![]() ΒΡ
ΒΡ![]() »ή“Κ÷–
»ή“Κ÷–![]() Υφ
Υφ![]() ΒΡ±δΜ·»γΆΦΥυ ΨΘ°
ΒΡ±δΜ·»γΆΦΥυ ΨΘ°

![]() ”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ
”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ![]() »ή“Κ÷–ΒΡΉΣΜ·Ζ¥”Π______________________________ Θ°
»ή“Κ÷–ΒΡΉΣΜ·Ζ¥”Π______________________________ Θ°
![]() ”…ΆΦΩ…÷ΣΘ§»ή“ΚΥα–‘‘ω¥σΘ§
”…ΆΦΩ…÷ΣΘ§»ή“ΚΥα–‘‘ω¥σΘ§![]() ΒΡΤΫΚβΉΣΜ·¬ __________________
ΒΡΤΫΚβΉΣΜ·¬ __________________ ![]() ΧνΓΑ‘ω¥σΓΑΦθ –ΓΓ±ΜρΓΑ≤Μ±δΓ±
ΧνΓΑ‘ω¥σΓΑΦθ –ΓΓ±ΜρΓΑ≤Μ±δΓ±![]() ΗυΨίAΒψ ΐΨίΘ§ΦΤΥψ≥ωΗΟΉΣΜ·Ζ¥”ΠΒΡΤΫΚβ≥Θ ΐΈΣ _______________ Θ°
ΗυΨίAΒψ ΐΨίΘ§ΦΤΥψ≥ωΗΟΉΣΜ·Ζ¥”ΠΒΡΤΫΚβ≥Θ ΐΈΣ _______________ Θ°
![]() …ΐΗΏΈ¬Ε»Θ§»ή“Κ÷–
…ΐΗΏΈ¬Ε»Θ§»ή“Κ÷–![]() ΒΡΤΫΚβΉΣΜ·¬ Φθ–ΓΘ§‘ρΗΟΖ¥”ΠΒΡ
ΒΡΤΫΚβΉΣΜ·¬ Φθ–ΓΘ§‘ρΗΟΖ¥”ΠΒΡ![]() ______
______ ![]() ΧνΓΑ¥σ”ΎΓ±ΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±
ΧνΓΑ¥σ”ΎΓ±ΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±![]() Θ°
Θ°
Θ®3Θ©‘ΎΜ·―ßΖ÷Έω÷–≤…”Ο![]() ΈΣ÷Η ΨΦΝΘ§“‘
ΈΣ÷Η ΨΦΝΘ§“‘![]() ±ξΉΦ»ή“ΚΒΈΕ®»ή“Κ÷–ΒΡ
±ξΉΦ»ή“ΚΒΈΕ®»ή“Κ÷–ΒΡ![]() Θ§άϊ”Ο
Θ§άϊ”Ο![]() ”κ
”κ![]() …ζ≥…Ή©Κλ…Ϊ≥ΝΒμΘ§÷Η ΨΒΫ¥οΒΈΕ®÷’Βψ
…ζ≥…Ή©Κλ…Ϊ≥ΝΒμΘ§÷Η ΨΒΫ¥οΒΈΕ®÷’Βψ![]() Β±»ή“Κ÷–
Β±»ή“Κ÷–![]() «ΓΚΟΆξ»Ϊ≥ΝΒμ
«ΓΚΟΆξ»Ϊ≥ΝΒμ![]() ≈®Ε»Β»”Ύ
≈®Ε»Β»”Ύ![]() ±Θ§»ή“Κ÷–
±Θ§»ή“Κ÷–![]() ΈΣ ______
ΈΣ ______ ![]() Θ§¥Υ ±»ή“Κ÷–
Θ§¥Υ ±»ή“Κ÷–![]() Β»”Ύ ______
Β»”Ύ ______ ![]() “―÷Σ
“―÷Σ![]() ΓΔAgClΒΡ
ΓΔAgClΒΡ![]() Ζ÷±πΈΣ
Ζ÷±πΈΣ![]() ΚΆ
ΚΆ![]()
Θ®4Θ©![]() ΦέΗθΒΡΜ·ΚœΈοΕΨ–‘Ϋœ¥σΘ§≥Θ”Ο
ΦέΗθΒΡΜ·ΚœΈοΕΨ–‘Ϋœ¥σΘ§≥Θ”Ο![]() ΫΪΖœ“Κ÷–ΒΡ
ΫΪΖœ“Κ÷–ΒΡ![]() ΜΙ‘≠≥…
ΜΙ‘≠≥…![]() Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ _________________________________________________ Θ°
Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ _________________________________________________ Θ°
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
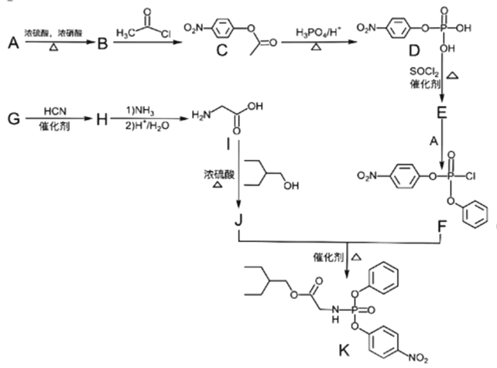
ΓΨΧβΡΩΓΩ“©Έο»πΒ¬ΈςΈΛΘ®RemdesivirΘ©)Ε‘2019Ρξ–¬–ΆΙΎΉ¥≤ΓΕΨΘ®2019-nCoVΘ©”–Οςœ‘“÷÷ΤΉς”ΟΘΜKΈΣ“©ΈοΚœ≥…ΒΡ÷–ΦδΧεΘ§ΤδΚœ≥…¬ΖœΏ»γœ¬ΘΚ
“―÷ΣΘΚ1. ![]()
2. ![]()
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©Φλ―ιA÷–ΙΌΡήΆ≈ΒΡΖΫΖ® «________________Θ§BΒΡΜ·―ßΟϊ≥ΤΈΣ_________ΓΘ
Θ®2Θ©CΓζDΒΡΖ¥”Πάύ–Ά «_____________Θ§JΘΪFΓζKΒΡΖ¥”Πάύ–Ά «___________ΓΘ
Θ®3Θ©I÷–ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ___________________Θ§FΒΡΖ÷Ή” Ϋ «_____________ΓΘ
Θ®4Θ©EΒΡΫαΙΙΦρ ΫΈΣ_________________________________ΓΘ
Θ®5Θ©”…G…ζ≥…HΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣ______________________________________ΓΘ
Θ®6Θ©X «CΆ§Ζ÷“λΙΙΧεΘ§–¥≥ω¬ζΉψœ¬Ν–ΧθΦΰΒΡXΒΡΫαΙΙΦρ Ϋ__________________ΓΘ
ΔΌ±ΫΜΖ…œΚ§”–œθΜυ«“±ΫΜΖ…œ÷Μ”–“Μ÷÷«β‘≠Ή”ΘΜ
ΔΎ”ωFeCl3»ή“ΚΖΔ…ζœ‘…ΪΖ¥”ΠΘΜ
Δέ1molΒΡX”κΉψΝΩΫπ τNaΖ¥”ΠΩ……ζ≥…2gH2 ΓΘ
Θ®7Θ©…ηΦΤ”…±ΫΦΉ¥ΦΈΣ‘≠Νœ÷Τ±ΗΜ·ΚœΈο ΓΘ
ΓΘ
___________________________________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΈ“Ιζ‘ΎΖ¥–ΥΖήΦΝΈ Χβ…œΒΡΦαΨωΝΔ≥Γ «÷ß≥÷ΓΑ»ΥΈΡΑ¬‘ΥΓ±ΒΡ÷Ί“ΣΧεœ÷ΓΘΡ≥÷÷–ΥΖήΦΝΒΡΫαΙΙ»γœ¬ΓΘΙΊ”ΎΥϋΒΡΥΒΖ®’ΐ»ΖΒΡ «(ΓΓΓΓ)
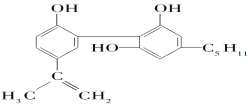
A.ΥϋΒΡΜ·―ß ΫΈΣC19H26O3
B.ΗΟΜ·ΚœΈο÷ΜΡήΖΔ…ζ»Γ¥ζΖ¥”Π
C.¥”ΫαΙΙ…œΩ¥Θ§Υϋ τ”Ύ¥Φάύ
D.¥”‘ΣΥΊΉι≥……œΩ¥Θ§ΥϋΩ…“‘‘Ύ―θΤχ÷–»Φ…’…ζ≥…CO2ΚΆΥ°
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΩΉ»Η ·¬ΧΒΡΫαΙΙΦρ Ϋ»γœ¬ΆΦΥυ ΨΘ§œ¬Ν–”–ΙΊΥΒΖ®’ΐ»ΖΒΡ «
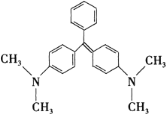
A.ΩΉ»Η ·¬ΧΒΡΖ÷Ή” ΫΈΣ C23H25N2
B.1 mol ΩΉ»Η ·¬Χ‘Ύ“ΜΕ®ΧθΦΰœ¬ΉνΕύΩ…”κ 6 mol H2 ΖΔ…ζΦ”≥…Ζ¥”Π
C.ΩΉ»Η ·¬ΧΉνΕύ”– 22 ΗωΧΦ‘≠Ή”Ι≤Οφ
D.ΩΉ»Η ·¬Χ±ΫΜΖ…œΒΡ“Μ¬»»Γ¥ζΈο”– 5 ÷÷
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΑ¥“‘œ¬ΉΑ÷ΟΆΦΫχ–– Β―ιΘ§Χν–¥”–ΙΊœ÷œσΦΑΜ·―ßΖΫ≥Χ ΫΓΘ

Θ®1Θ©A÷–œ÷œσ «____________________Θ§
Θ®2Θ©B÷–≈®ΝρΥαΒΡΉς”Ο «_______________
Θ®3Θ©C÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ______________Θ§
Θ®4Θ©D÷–œ÷œσ «_______________ΘΜΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «__________________________Θ§
E÷– ’Φ·ΒΡΤχΧε «____________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ»γΆΦ «‘ΣΥΊMΒΡΦέάύΕΰΈ§ΆΦΓΘΤδ÷–A «“Μ÷÷―ΈΘ§EΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ±»DΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ¥σ16Θ§AΚΆB «Κ§ΉνΒΆΦέM‘ΣΥΊΒΡΝΫ÷÷Μ·ΚœΈοΘ§Β±xΈΣ“Μ÷÷«ΩΦν ±Θ§”–»γœ¬ΉΣΜ·ΙΊœΒΓΘœ¬Ν–ΥΒΖ®≤Μ’ΐ»ΖΒΡ «Θ® Θ©
A.Έο÷ B”ω…ΌΝΩΒΡ¬»ΤχΜα≤ζ…ζΑΉ―Χ
B.FΒΡ≈®»ή“ΚΩ…“‘”ΟΧζ÷Τ»ίΤς ΔΖ≈
C.E”κΥ°Ζ¥”ΠΩ……ζ≥…F
D.Έο÷ DΡήΙΜ±ΜxΆξ»ΪΈϋ ’
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com