
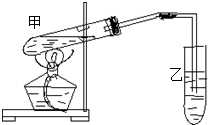
 ��ͼΪijͬѧ��Ƶ�������������ʵ��װ��ͼ��
��ͼΪijͬѧ��Ƶ�������������ʵ��װ��ͼ��| 1 | 3 |
| 1 |
| 3 |
| 1 |
| 3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
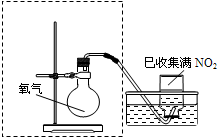 ijС�����ʵ�飬̽��NO2��ˮ���գ�����ﵽ����NO2������ȫ������ �ڼ���ƿ�в��������御�����٣�
ijС�����ʵ�飬̽��NO2��ˮ���գ�����ﵽ����NO2������ȫ������ �ڼ���ƿ�в��������御�����٣�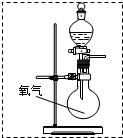

| ʵ�鲽�� | ʵ������ | ��ѧ����ʽ | |
| �� | ���ռ���NO2�ļ���ƿ���� ��ˮ���� |
||
| �� | ��������ɫ��ɺ���ɫ�� ���ֱ����ɫ��Һ��� ������ | ||
| �� | ��������������ͬ����� ƿ��ֻ�����������壬Һ �弸������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
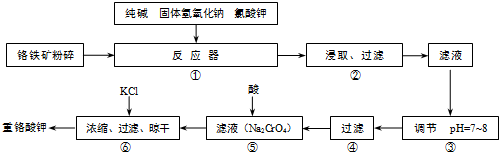
| �������� | ʵ������ | ���� |
| ��ȡ�������������Թ��У�������ϡHCl�����裬���ã� | �Թ������й������� | ����Ϊ |
| �ڲ����ٺ��ˣ�����Һ�м�������� |
�к��ɫ�������� | ����ΪFe��OH��3 |
| �۽������ں�ĺ��ɫ������ȥ������Һ��ͨ������CO2�� | ������ΪAl��OH��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���㣺
���㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
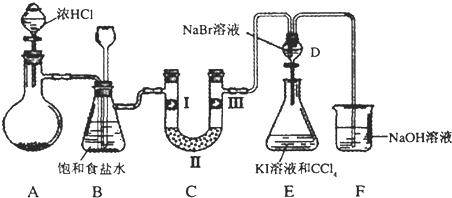
| a | b | c | d | |
| I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | �轺 | ��ʯ�� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com