
����Ŀ����Դ����������ͷ�չ����Ҫ֧��������Ҫ��ش��������⡣
(1)����˵���д������______��

A�����������Դ
B�����ܣ�H2���ڶ�����Դ��ˮ����һ����Դ
C��ú��ʯ�����ڻ�ʯȼ�ϣ�ȼ�ջ������������
D��������ֵ�ߣ��Ի�������Ⱦ
(2)�ҹ�Ŀǰʹ�õ���Ҫ��Դ�ǻ�ʯȼ�ϣ��������ҹ���Դ�����������˾���Դ��������ͼ��
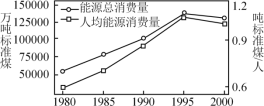
�����ҹ���ú������������ú���ڲ���������Դ��Ѱ������Դ����Դ�о�����Ҫ����֮һ�����в���������Դ����______��
A��̫���� B������ C�������� D��������
E���������� F��ʯ�� G����Ȼ��
����֪��1 kgú��Լ����2.9��104 kJ����������������һ������õķ����൱��5��1014 kw��h�ĵ��ܡ��Լ��㣬һ��ķ�������ȫ�����ã����൱�ڽ�ʡ______��ȼú��(��֪��1 kw��h��3.6��103 kJ)
���𰸡�A FG 6.2��1010
��������
�����Դ������ɫ��Դ����ָ���ŷ���Ⱦ��ܹ�ֱ�����������������Դ�����������ܺ͡���������Դ��������Դһ����ָ���¼��������ϼ��Կ������õĿ�������Դ������̫���ܡ��������ܡ����ܡ������ܡ������ܡ����ܡ������ܵȡ�
��1��A�������Դ������ɫ��Դ����ָ���ŷ���Ⱦ��ܹ�ֱ�����������������Դ�����������ܺ͡���������Դ������˻�ʯȼ�Ϻͽ�¯�����ܳ�Ϊ�����Դ��A�����
B�����ܣ�H2����һ����Դ�Ʊ����õĶ�����Դ��ˮ����һ����Դ��B����ȷ��
C��ú��ʯ�͡���Ȼ�������ڻ�ʯȼ�ϣ�ȼ��ʱ������������������̼��C����ȷ��
D��������ֵ�ߣ��Ի�������Ⱦ����һ�����������Դ��D����ȷ��
��ѡA��
(2)
������Դһ����ָ���¼��������ϼ��Կ������õĿ�������Դ������̫���ܡ��������ܡ����ܡ������ܡ������ܡ����ܡ������ܵȣ���ú��ʯ�͡���Ȼ�������ڻ�ʯȼ�ϲ���������Դ����˲���������Դ��Ӧѡ��FG��
����֪��1 kgú��Լ����2.9��104 kJ��һ������õķ����൱��5��1014 kw��h�ĵ��ܣ�����1 kw��h��3.6��103 kJ���ɻ����һ������õķ����ṩ������Ϊ5��1014 kw��h��3.6��103 kJ=1.8��1018 kJ���൱��ȼú������![]() = 6.2��1013 kg=6.2��1010t��
= 6.2��1013 kg=6.2��1010t��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ���ѧ�ҳɹ�ʵ�ּ����ڴ��������������£�һ����Ч������ϩ�������������Ȼ�ѧƷ��Ϊ��Ȼ������������һ�������Լ������Լ���Ϊԭ�Ϻϳɲ��ֻ�����Ʒ�������£����ַ�Ӧ��������ȥ����

��1��E ������Ϊ____��B���ʵĽṹ��ʽ��______��
��2�������ۡ���ת����Ӧ�У�����ȡ����Ӧ����______���÷�Ӧ�����д����
��3��д����Ӧ�ߵķ�Ӧ����ʽ��______��
��4����ͼΪʵ������ȡE��װ��ͼ��ͼ��a�Լ�Ϊ_______��

��5��ijͬѧ���Թ�b�м���6.0������������Ҵ������ʵ�����ʹ��Ӧ��ֽ��У����������Թ�b���յ�3.0�����ᣬ���ͬѧ�ڱ���ʵ�����Ƶ������������������Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ��֣�H2S�Ǽ�NO��CO֮��ĵ�����������ϵ�����źŷ��ӣ������в���������źŴ��ݡ�����Ѫ�ܼ����Ѫѹ�Ĺ��ܡ��ش��������⣺
��1��������ʵ�У����ܱȽ��������������������ǿ������________(����)��
A�����������̼��������Һ��Ӧ�������������
B��������ĵ�������������ͬŨ�ȵ�������
C��0.10 mol��L��1����������������pH�ֱ�Ϊ4.5��2.1
D��������Ļ�ԭ��ǿ��������
��2����ͼ��ͨ���Ȼ�ѧѭ���ڽϵ��¶�����ˮ������ֽ��Ʊ������ķ�Ӧϵͳԭ����

ͨ�����㣬��֪ϵͳ(��)��ϵͳ(��)������Ȼ�ѧ����ʽ�ֱ�Ϊ_______���Ƶõ���H2�����������ٵ���________��
��3��H2S��CO2�ڸ����·�����Ӧ��H2S(g)��CO2(g)![]() COS(g)��H2O(g)����610 Kʱ����0.10 mol CO2��0.40 mol H2S����2.5 L�Ŀո�ƿ�У���Ӧƽ���ˮ�����ʵ�������Ϊ0.02��
COS(g)��H2O(g)����610 Kʱ����0.10 mol CO2��0.40 mol H2S����2.5 L�Ŀո�ƿ�У���Ӧƽ���ˮ�����ʵ�������Ϊ0.02��
��H2S��ƽ��ת������1��________%����Ӧƽ�ⳣ��K��________��
����620 K�ظ�ʵ�飬ƽ���ˮ�����ʵ�������Ϊ0.03��H2S��ת������2_______��1���÷�Ӧ����H________0��(����>����<����������)
����Ӧ�����ٷֱ�����������壬��ʹH2Sת�����������________(����)��
A��H2S B��CO2 C��COS D��N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ijѧ���Ļ�ѧʵ�鱨���������¼�¼����ʵ�����ݺ����ģ� ��
A.��100 mL��Ͳ��ȡ5.26 mLϡ������Һ
B.��������ƽ��ȡ11.7 g CuO��ĩ
C.�ù㷺pH��ֽ�����Һ��pHΪ3.5
D.�¶ȼ�����ʾ�����¶���Ϊ25.68 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Fe���ճ���������õĽ���֮һ���ش��������⣺
(1)�����ڳ�ʪ�����е����ܵ����������λ�ԭ��(�û�ԭ��������滷����pHΪ7��8֮��)�����£��ܱ�SO42-��ʴ����绯ѧ��ʴԭ������ͼ��ʾ��д�������ĵ缫��Ӧʽ___________________��
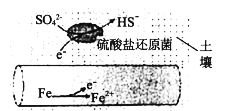
(2)��1800Kʱ��2Fe(s)+3/2O2(g)=Fe2O3(s) H=-354.2kJ/mol��3Fe(s)+2O2(g)=Fe3O4(s) H=-550.9kJ/mol��Ӧ��2Fe3O4(s)+1/2O2(g) ===3Fe2O3(s)��HΪ_____ kJ��mol1�������������ڳ��������ļ���ƿ�з�Ӧ����Fe2O3_____(������������������)�Է����С�
(3)Fe3+��I����ˮ��Һ�еķ�Ӧ���£�2I��+2Fe3+![]() 2Fe2+ +I2(��ˮ��Һ��)��
2Fe2+ +I2(��ˮ��Һ��)��
��298Kʱ����5mL 0.1molL1 ��KI��Һ�еμ�0.1molL1 FeCl3��Һ���õ�c(Fe2+)�����FeCl3 ��Һ�����ϵ����ͼ��ʾ��
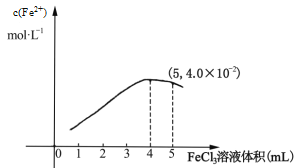
���¶��µμ�5mL FeCl3��Һʱ��Fe3+��ƽ��ת����=_____%��ƽ�ⳣ��K=_____����Ҫ���Fe3+��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��________________________��
�����Ѿ��ﵽƽ���������Ӧ��ϵ�У����뱽��I2������ȡ�������¶Ȳ��䣬��Ӧ����_____ (����������������С������������)����ʱ��(��)_____��(��)(��������������С��������������)��
����(��)��Fe3+��I��Ũ�ȹ�ϵΪ��=kc(I��)mc(Fe3+)n(kΪ����)
c(I)molL1 | c(Fe3+)molL1 | �� (molL1s1 ) | |
(1) | 0.20 | 0.80 | 0.032k |
(2) | 0.60 | 0.40 | 0.144k |
(3) | 0.80 | 0.20 | 0.128k |
ͨ�������������ݼ����֪������=kc(I��)mc(Fe3+)n �У�m��n��ֵΪ_____(����ĸ����)��
A��m=1��n=1�� B��m=2��n=1 ��C��m=2��n=2 ��D��m=1��n=2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Ӧ�����з������ʱ仯��ͬʱ���������������ı仯�����������ı仯������������ʽ���ֳ�����������Ӧ�ȣ����ڷ�Ӧ�������ͬ����Ӧ�ȿ��Է�Ϊ�����֣���ȼ���Ⱥ��к��ȵȡ�
(1)������H��ʾ����ȼ���ȵ��� ______ ����ʾ�����к��ȵ��� ______ (������H1��������H2��������H3����)��
A��2H2(g)+O2(g)�T2H2O(l)��H1
B��C(s)+1/2O2(g)�TCO(g)��H2
C��CH4(g)+2O2(g)�TCO2(g)+2H2O(g)��H3
D��C(s)+O2(g)�TCO2(g)��H4
E��C6H12O6(s)+6O2(g)�T6CO2(g)+6H2O(l)��H5��
F��NaOH(aq)+HCl(aq)�TNaCl(aq)+H2O(l)��H6
G��2NaOH(aq)+H2SO4(aq)�TNa2SO4(aq)+2H2O(l)��H7
H��CH3COOH(aq)+NaOH(aq)�TCH3COONa(aq)+H2O(l)��H8
(2)������ʵ��д�����з�Ӧ���Ȼ�ѧ����ʽ��
����25�桢101kPa�£�1gCH3OHȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ _______________________��
��1.00L1.00mol/LH2SO4��Һ��2.00L1.00mol/LNaOH��Һ��ȫ��Ӧ���ų�114.6kJ����������ʾ���к��ȵ��Ȼ�ѧ����ʽΪ ________________________��
����֪��1molH-H����1molN-H����1molN��N���ֱ���Ҫ��������436kJ��391kJ�� 946kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ _______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������㷺����ʳƷ��ζ���� ���㾫�� ���ϣ� �ϳ���·���£�
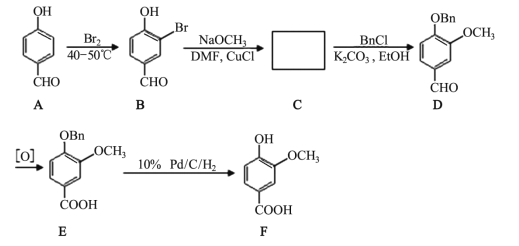
��1��C �Ľṹ��ʽ��_____�� B ת��Ϊ C �ķ�Ӧ����Ϊ_____��
��2��A �к��еĹ�����������_____��
��3��B �ķ���ʽ��_____��
��4���� B �е��� FeCl3��Һ�� ��������_____��
��5���� F ��������ͬ�ķ����廯�����ͬ���칹����_____�֡�
��6��д�� F ���Ҵ�����������Ӧ�ķ���ʽ_____��
��7��д���� �ĺϳ���·ͼ_____��
�ĺϳ���·ͼ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25�桢101 kPa��,1g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ,�����Ȼ�ѧ����ʽ��д��ȷ����()
A. CH3OH(l)+3/2O2(g)![]() CO2(g)+2H2O(l) ��H=+725.76 kJ��mol-1
CO2(g)+2H2O(l) ��H=+725.76 kJ��mol-1
B. 2CH3OH(l)+3O2(g)![]() 2CO2(g)+4H2O(l) ��H=-1 451.52 kJ��mol-1
2CO2(g)+4H2O(l) ��H=-1 451.52 kJ��mol-1
C. 2CH3OH(l)+3O2(g)![]() 2CO2(g)+4H2O(l) ��H=-725.76 kJ��mol-1
2CO2(g)+4H2O(l) ��H=-725.76 kJ��mol-1
D. 2CH3OH(l)+3O2(g)![]() 2CO2(g)+4H2O(l)��H=+1451.52 kJ��mol-1
2CO2(g)+4H2O(l)��H=+1451.52 kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȸʯ����Ҫ�ɷ���Cu2(OH)2CO3����Fe2O3��FeCO3��Al2O3��SiO2���ʣ�����ҵ���ÿ�ȸʯ�Ʊ�����ͭ�ĵ�һ�����ù����������ܽⲢ���ˡ������£��ֱ�ȡ��Һ�������м���ָ�����ʣ���Ӧ�����Һ�д������ڵ���������ȷ����
A. ���������ˮ��Fe3+��NH4+��SO42����OH��
B. �������NaClO��Һ��Fe2+��Na+��ClO����SO42��
C. �������NaOH��Һ��Na+��AlO2����SO42����OH��
D. �������NaHCO3��Һ��Na+��Al3+��SO42����HCO3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com