
��֪��P4(s)��6Cl2(g)��4PCl3(g) ��H��akJ��mol��1
P4(s)��10Cl2(g)��4PCl5(g) ��H�� bkJ��mol��1
P4������������ṹ��PCl5��P��Cl���ļ���ΪckJ��mol��1,PCl3��P��Cl���ļ���Ϊ1.2ckJ��mol��1
����������ȷ����( )
| A��P��P���ļ��ܴ���P��Cl���ļ��� |
| B������Cl2(g)��PCl3(g)��PCl5(s)�ķ�Ӧ�Ȧ�H |
C��Cl��Cl���ļ���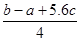 kJ��mol��1 kJ��mol��1 |
D��P��P���ļ���Ϊ kJ��mol��1 kJ��mol��1 |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����25�桢101kPa״���£�4g����������������Ӧ����1molˮ��������241.8kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��
��1����25�桢101kPa״���£�4g����������������Ӧ����1molˮ��������241.8kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��| ʵ�� ��� |
���� ����/g |
���� ״̬ |
C��H2SO4�� /mol?L-1 |
V��H2SO4�� /mL |
��Һ�¶�/�� | ������ʧ ��ʱ��/s | |
| ��Ӧǰ | ��Ӧ�� | ||||||
| 1 | 0.10 | ˿ | 0.5 | 50 | 20 | 34 | 500 |
| 2 | 0.10 | ��ĩ | 0.5 | 50 | 20 | 35 | 50 |
| 3 | 0.10 | ˿ | 0.7 | 50 | 20 | 36 | 250 |
| 4 | 0.10 | ˿ | 0.8 | 50 | 20 | 35 | 200 |
| 5 | 0.10 | ��ĩ | 0.8 | 50 | 20 | 36 | 25 |
| 6 | 0.10 | ˿ | 1.0 | 50 | 20 | 35 | 125 |
| 7 | 0.10 | ˿ | 1.0 | 50 | 35 | 50 | 50 |
| 8 | 0.10 | ˿ | 1.1 | 50 | 20 | 34 | 100 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1������ʯ����Ҫ�ɷ�Ca5��PO4��
��������Ӧ�У����������������___________________________________��
����֪��ͬ�����£�
4Ca5��PO4��
2Ca3��PO4��2��s��+
SiO2��s��+CaO��s��====CaSiO3��s�� ��H3
�æ�H1����H2�ͦ�H3��ʾ��H����H��_____________________________��
��2�������������Ϊ����������ӣ�����ṹʽ����ͼ��֮����ȥ����ˮ���ӵIJ����ṹʽΪ________________________�����������ƣ��׳ơ����ơ����dz��õ�ˮ���������仯ѧʽΪ___________________________��

��3���������ƣ�NaH2PO2�������ڻ�ѧ������
��NaH2PO2��PԪ�صĻ��ϼ�Ϊ________________________��
�ڻ�ѧ��������Һ�к���Ni2+��![]() �������Ե������·���������Ӧ��
�������Ե������·���������Ӧ��
(a)__________Ni2++__________![]() +__________
+__________![]() __________Ni+________
__________Ni+________![]() +_______
+_______
��b��6![]() +2H+====2P+4
+2H+====2P+4![]() +3H2��
+3H2��
���������д������ƽ��Ӧʽ��a����
�����â��з�Ӧ�������϶Ƽ�����������Ͻ𣬴Ӷ��ﵽ��ѧ������Ŀ�ģ�����һ�ֳ����Ļ�ѧ�ơ�������·���Ƚϻ�ѧ�����ơ�
�����ϵIJ�ͬ�㣺_______________��ԭ���ϵ���ͬ�㣺_______________����ѧ�Ƶ��ŵ㣺_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�찲��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
�Ͼ�ӡˢ��·��Ļ������ÿ�ʵ����Դ��������������Ⱦ���Ͼ�ӡˢ��·�徭������룬�ܵõ��ǽ�����ĩ�ͽ�����ĩ��
��1�����д���ӡˢ��·��ǽ�����ĩ�ķ����У������ϻ�������������� ������ĸ����
A�����ѽ��γ�ȼ��B��¶����� C����Ϊ�л����Ͻ������ϵ�ԭ��D��ֱ������
��2����H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
Cu(s)��2H��(aq)=Cu2��(aq)��H2(g) ��H=64.39kJ��mol��1
2H2O2(l)=2H2O(l)��O2(g) ��H=��196.46kJ��mol��1
H2(g)��1/2O2(g)=H2O(l)
��H=��285.84kJ��mol��1
�� H2SO4��Һ��Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ ��
��3����������������ͬ��ӡˢ��·��Ľ�����ĩ��10�GH2O2��3.0mol��L��1H2SO4�Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
|
�¶ȣ��棩 |
20 |
30 |
40 |
50 |
60 |
70 |
80 |
|
ͭƽ���ܽ����ʣ���10-3 mol��L-1��min-1�� |
7.34 |
8.01 |
9.25 |
7.98 |
7.24 |
6.73 |
5.76 |
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
��4�����ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ�� ��
��5�� ��֪��ͬ�����£�
4Ca5(PO4)3F(s)+3SiO2(s)=6Ca3(PO4)2(s)+2CaSiO3(s)+SiF4(g) ����H1
2Ca3(PO4)2(s)+10C(s)=P4(g)+6CaO(s)+10CO(g)����H2
SiO2(s)+CaO(s)=CaSiO3(s) ����H3
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) �� H
H
�á�H1����H2�͡�H3��ʾ H��
H�� H=
��
H=
��
��6����֪1 g FeS2(s)��ȫȼ�����ɷų�7.1 kJ������FeS2ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ѧ�걱���г�����������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
���ݱ�����Ϣ�ش��������⡣
|
Ԫ�� |
Si |
P |
S |
Cl |
|
���������� ��Ӧ������ |
���� |
�������������ܷ�Ӧ |
���� |
���ջ��ȼʱ������ը������ |
��1��S��Ԫ�����ڱ��е�λ���� ��
��2�����ݱ�����Ϣ��֪��Si��P��S��Cl ����Ԫ�صĵķǽ�����������ǿ����ԭ�ӽṹ����ԭ��ͬ����Ԫ�ص��Ӳ�����ͬ���������ң� ��ԭ�Ӱ뾶��С���õ�����������ǿ��
��3��25��ʱ����������Ԫ�صĵ�����������Ӧ����l mol��̬�⻯��ķ�Ӧ�����£�
a��+34.3 kJ��mol-1 b��+9.3 kJ��mol-1 c��−20.6 kJ��mol-1 d��−92.3 kJ��mol-1
��д����̬���ף�P4����H2��Ӧ������̬�⻯����Ȼ�ѧ����ʽ ��
��4��̽��ͬ����Ԫ�����ʵ�һЩ��ͬ���ɣ���ѧϰ��ѧ����Ҫ����֮һ����֪����Se��������������Ԫ�أ��䲿����Ϣ��ͼ��
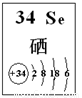
�������й�˵����ȷ���� ������ĸ����
a. ԭ�Ӱ뾶��Se��S��P b. �ȶ��ԣ�H2Se��H2S
c. ��Ϊ����H2Se��HCl�����Էǽ�����Se��Cl
d. SeO2����������������ռ���Һ��Ӧ
�����±����г���H2SeO3���ֲ�ͬ��ѧ���ʵ��Ʋ⣬������д����Ӧ�Ļ�ѧ����ʽ��
|
��� |
�����Ʋ� |
��ѧ����ʽ |
|
1 |
������ |
H2SeO3��4HI=Se����2I2��3H2O |
|
2 |
|
|
|
3 |
|
|
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com