
ЁОЬтФПЁПгУ0.1 mol/LЕФNaOHШмвКЗжБ№ЕЮЖЈЬхЛ§ОљЮЊ20mLЁЂХЈЖШОљЮЊ0.1 mol/LЕФHClШмвККЭHXШмвКЃЌШмвКЕФpHЫцМгШы NaOHШмвКЬхЛ§ЕФБфЛЏШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
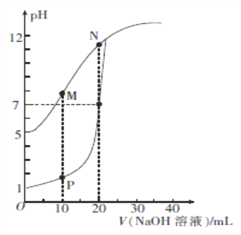
A. HXЕФЕчРыЦНКтГЃЪ§KaдМЮЊ1ЁС10-5
B. MЕуЖдгІШмвКжа:c(HX)< c (X-)
C. PЕуЖдгІШмвКжа: c(Cl-)=0.05mol/L
D. NЕуЖдгІШмвКжа:c(Na+)ЃОc(X-)ЃОc(OH-) ЃОc(H+)
ЁОД№АИЁПD
ЁОНтЮіЁПA.ОнЭМПЩжЊЃЌвђHClЪЧЧПЫсЃЌ0.1mol/LЕФHClШмвКpH=1ЃЌдђ0.1mol/LЕФHXШмвКpH=5ЃЌHXЪЧШѕЫсЃЌHXЕФЕчРыЦНКтГЃЪ§Ka=![]() =1ЁС10-9ЃЌЙЪAДэЮѓЃЛB.MЕуЪБМгШыNaOHШмвКЕФЬхЛ§ЮЊ10mLЃЌЫљЕУШмвКЮЊЕШХЈЖШЕФHXКЭNaXЕФЛьКЯШмвКЃЌДЫЪБpHЃО7ЃЌШмвКГЪМюадЃЌЫЕУїXЃЕФЫЎНтГЬЖШДѓгкHXЕФЕчРыГЬЖШЃЌдђc(HX)ЃОc (XЃ)ЃЌЙЪBДэЮѓЃЛC.pЕуЪБМгШыNaOHШмвКЕФЬхЛ§ЮЊ10mLЃЌЫљЕУШмвКжаc(ClЃ)=
=1ЁС10-9ЃЌЙЪAДэЮѓЃЛB.MЕуЪБМгШыNaOHШмвКЕФЬхЛ§ЮЊ10mLЃЌЫљЕУШмвКЮЊЕШХЈЖШЕФHXКЭNaXЕФЛьКЯШмвКЃЌДЫЪБpHЃО7ЃЌШмвКГЪМюадЃЌЫЕУїXЃЕФЫЎНтГЬЖШДѓгкHXЕФЕчРыГЬЖШЃЌдђc(HX)ЃОc (XЃ)ЃЌЙЪBДэЮѓЃЛC.pЕуЪБМгШыNaOHШмвКЕФЬхЛ§ЮЊ10mLЃЌЫљЕУШмвКжаc(ClЃ)= ![]() =0.067mol/LЃЌЙЪCДэЮѓЃЛD.NЕуЪБМгШыNaOHШмвКЕФЬхЛ§ЮЊ20mLЃЌHXКЭNaOHЧЁКУЭъШЋЗДгІЕУNaXШмвКЃЌXЃЗЂЩњЫЎНтЗДгІЪЙШмвКГЪМюадЃЌдђc(Na+)ЃОc(XЃ)ЃОc(OHЃ) ЃОc(H+)ЃЌЙЪDе§ШЗЃЛД№АИбЁDЁЃ
=0.067mol/LЃЌЙЪCДэЮѓЃЛD.NЕуЪБМгШыNaOHШмвКЕФЬхЛ§ЮЊ20mLЃЌHXКЭNaOHЧЁКУЭъШЋЗДгІЕУNaXШмвКЃЌXЃЗЂЩњЫЎНтЗДгІЪЙШмвКГЪМюадЃЌдђc(Na+)ЃОc(XЃ)ЃОc(OHЃ) ЃОc(H+)ЃЌЙЪDе§ШЗЃЛД№АИбЁDЁЃ


 ОйвЛЗДШ§ЭЌВНЧЩНВОЋСЗЯЕСаД№АИ
ОйвЛЗДШ§ЭЌВНЧЩНВОЋСЗЯЕСаД№АИ ПкЫугыгІгУЬтПЈЯЕСаД№АИ
ПкЫугыгІгУЬтПЈЯЕСаД№АИ УћЪІЕуОІзжДЪОфЖЮЦЊЯЕСаД№АИ
УћЪІЕуОІзжДЪОфЖЮЦЊЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЛЏбЇгУгяБэЪОе§ШЗЕФЪЧ
A. СђЫсбЧЬњЕФЕчРыЗНГЬЪНЃКFe2ЃЈSO4ЃЉ3 ЃН2Fe3+ЃЋ3SO42Ѓ
B. H2SO4ЕФЕчРыЗНГЬЪНЃКH2SO4ЃНH2+ + SO42-
C. жЪзгЪ§ЮЊ6ЃЌжазгЪ§ЮЊ7ЕФЮЂСЃЃК76C
D. ФЦРызгЕФНсЙЙЪОвтЭМЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈжаДэЮѓЕФЪЧ
A. ЮЊСЫГ§ШЅMgCl2ЫсадШмвКжаЕФFe3+ЃЌПЩдкМгШШЬѕМўЯТМгШыNaOHШмвК
B. ЯђK2Cr2O7ШмвКжаМгШызуСПХЈNaOHШмвКЃЌШмвКгЩГШЩЋБфГЩЛЦЩЋ
C. КЌгаKIКЭH2SO4ЕФЛьКЯШмвКЗХжУвЛЖЮЪБМфКѓЃЌМгШыЕэЗлШмвКЃЌШмвКБфРЖ
D. бЮМюЕиЃЈКЌНЯЖрNaClЁЂNa2CO3ЃЉжаМгШыЪЪСПЕФЪЏИрЃЈCaSO4ЃЉПЩвдНЕЕЭЭСШРЕФМюад
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЁЂEЁЂFЮЊЧАЫФжмЦкдзгађЪ§вРДЮдіДѓЕФСљжждЊЫиЃЌAЁЂCЁЂDдзгОљгаСНИіЮДГЩЖдЕчзгЃЌAЁЂBЁЂCЭЌжмЦкЃЌAгыDЁЂBгыFЗжБ№ЭЌжїзхЃЌEЪЧЩњЛюжагУСПзюДѓЕФН№ЪєЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉEБШНЯЮШЖЈЕФРызгКЫЭтЕчзгХХВМЪН_____________________________ЃЎ
ЃЈ2ЃЉAЁЂBЁЂCЕФЕквЛЕчРыФмгЩаЁЕНДѓЕФЫГађЮЊ_________________![]() гУдЊЫиЗћКХБэЪО
гУдЊЫиЗћКХБэЪО![]() ЁЂBЁЂCЕФМђЕЅЧтЛЏЮяжазювзЦћЛЏЕФЮяжЪЕФЛЏбЇЪН___________ЃЎ
ЁЂBЁЂCЕФМђЕЅЧтЛЏЮяжазювзЦћЛЏЕФЮяжЪЕФЛЏбЇЪН___________ЃЎ
ЃЈ3ЃЉCгыDаЮГЩЕФЮяжЪЕФОЇЬхРраЭЪЧ____________ЃЌITВњвЕжаИУОЇЬхгУгкЩњВњ____________ЃЎ
ЃЈ4ЃЉгЩAЁЂBЁЂCШ§жждЊЫижаЕФвЛжжЛђСНжждЊЫиаЮГЩЕФЗжзгжаЃЌгаЕФЛЅЮЊЕШЕчзгЬхЃЌаДГіЦфжавЛзщЕШЕчзгЬхЕФЛЏбЇЪНЃК______![]() ВЂаДГіЖдгІЕФНсЙЙЪН_______________ЃЎ
ВЂаДГіЖдгІЕФНсЙЙЪН_______________ЃЎ
ЃЈ5ЃЉBЕФЕЅжЪОЇАћгы![]() ЕФЯрЫЦЃЌдђвЛИіОЇАћжаКЌBЕФдзгИіЪ§ЮЊ____
ЕФЯрЫЦЃЌдђвЛИіОЇАћжаКЌBЕФдзгИіЪ§ЮЊ____![]() гыЧтаЮГЩЕФЗжзгПеМфЙЙаЭЪЧ_______ЃЎ
гыЧтаЮГЩЕФЗжзгПеМфЙЙаЭЪЧ_______ЃЎ
ЃЈ6ЃЉЁАХјЫЊЁБЪЧвЛжжКЌCЃЌFЕФЛЏКЯЮяЃЌЦфЗжзгНсЙЙШчЭМ1ЫљЪОЃЌИУЛЏКЯЮяЕФЗжзгЪНЮЊF4C6ЃЌFдзгВЩШЁ______дгЛЏЃЛCЃЌDЃЌEзщГЩЕФЛЏКЯЮяЕФОЇАћШчЭМ2ЃЌЦфОЇАћВЮЪ§ЮЊa pmЃЌдђЦфУмЖШЮЊ___________________g/cm3ЃЈСаГіЪНзгМДПЩЃЌАЂЗќйЄЕТТоГЃЪ§ЮЊNAmol-1ЃЉЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗХЩфадЭЌЮЛЫиюи16667HoЕФдзгКЫФкЕФжазгЪ§гыКЫЭтЕчзгЪ§жЎВюЪЧЃЈЁЁЁЁЃЉ
A.99
B.67
C.32
D.166
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП![]() єЪЛљИ§ЫсЪЧвЛжжживЊЕФЛЏЙЄжаМфЬхЃЌЯТУцКЯГЩЫќЕФСїГЬЭМЃК
єЪЛљИ§ЫсЪЧвЛжжживЊЕФЛЏЙЄжаМфЬхЃЌЯТУцКЯГЩЫќЕФСїГЬЭМЃК
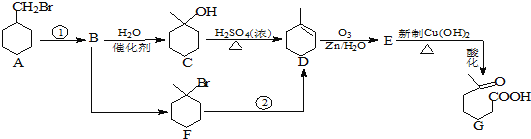
вбжЊЃК![]()
![]()
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЗДгІ![]() ЕФЬѕМўЪЧ _______________ ЃЛ1molЛЏКЯЮяCЭъШЋШМЩеашвЊЯћКФO2 ___________ mol
ЕФЬѕМўЪЧ _______________ ЃЛ1molЛЏКЯЮяCЭъШЋШМЩеашвЊЯћКФO2 ___________ mol
ЃЈ2ЃЉGЫљКЌЕФЙйФмЭХЕФУћГЦЪЧ _____________________ ЃЛ
ЃЈ3ЃЉЯТСаЫЕЗЈжае§ШЗЕФЪЧ ______________ ЃК
![]() гызуСПЕФNaЗДгІЩњГЩ
гызуСПЕФNaЗДгІЩњГЩ![]()
![]() ФмБЛДпЛЏбѕЛЏГЩЭЊ
ФмБЛДпЛЏбѕЛЏГЩЭЊ
![]() дкNiДпЛЏЯТ1molGзюЖржЛФмгы
дкNiДпЛЏЯТ1molGзюЖржЛФмгы![]() МгГЩ
МгГЩ ![]() ФмЗЂЩњЯћШЅЗДгІЩњГЩСНжжВЛЭЌЯЉЬў
ФмЗЂЩњЯћШЅЗДгІЩњГЩСНжжВЛЭЌЯЉЬў
ЃЈ4ЃЉEгыаТжЦ![]() ЕФЛЏбЇЗНГЬЪНЮЊ _________________ЁЃ
ЕФЛЏбЇЗНГЬЪНЮЊ _________________ЁЃ
ЃЈ5ЃЉGЕФЭЌЗжвьЙЙЬхгаЖржжЃЌЧыаДГіЗћКЯЯТСавЊЧѓЕФЭЌЗжвьЙЙЬхЃК___________________________________________________
ЂйНсЙЙжаКЌга ЂкЪєгкѕЅРр ЂлФмЗЂЩњвјОЕЗДгІ
ЂкЪєгкѕЅРр ЂлФмЗЂЩњвјОЕЗДгІ
ЃЈ6ЃЉаДГівд ЮЊдСЯжЦБИ
ЮЊдСЯжЦБИ ЕФКЯГЩТЗЯпСїГЬЭМ
ЕФКЯГЩТЗЯпСїГЬЭМ![]() ЮоЛњЪдМСШЮгУ
ЮоЛњЪдМСШЮгУ![]() ЃЌВЂзЂУїЗДгІЬѕМўЁЃ_________________
ЃЌВЂзЂУїЗДгІЬѕМўЁЃ_________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгаМзЁЂввЁЂБћШ§жжЕШЬхЛ§ЕШЮяжЪЕФСПХЈЖШЕФNaOHШмвКЁЃШєНЋМзеєЗЂвЛАыЫЎЃЌдкввжаЭЈШыЩйСПCO2ЃЌБћВЛБфЃЌШЛКѓгУЕШХЈЖШЕФH2SO4ШмвКЕЮЖЈЃЌгУМзЛљГШзїжИЪОМСЁЃЭъШЋЗДгІКѓЃЌЫљашШмвКЬхЛ§ЪЧЃЈ ЃЉ
A.Мз=Бћ>вв B.Бћ>вв>Мз C.вв>Бћ>Мз D.Мз=вв=Бћ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮЊСЫЬНОПАБЦјМААБЫЎЕФЛЙдадЃЌФГаЫШЄаЁзщЭЌбЇЩшМЦСЫвдЯТЬНОПЛюЖЏЁЃ
I.ЬНОПАБЦјЕФЛЙдад
ИУаЫШЄаЁзщЭЌбЇРћгУвдЯТзАжУ(МаГжЃЌМгШШвЧЦїТд)ЬНОПТШЦјгыАБЦјЕФЗДгІЃЌЦфжаAЁЂFЗжБ№ЮЊТШЦјКЭАБЦјЕФЗЂЩњзАжУЃЌBЮЊДПОЛИЩдяЕФТШЦјгыАБЦјЗДгІЕФзАжУЁЃ
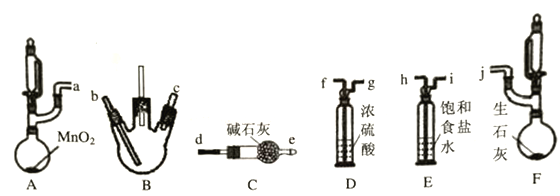
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЩЯЪізАжУНгПкЕФСЌНгЫГађЮЊaНгhЁЂiНгfЁЂgНг___ЁЂ____Нг___ЁЂ____НгjЃЌЦфжазАжУDЕФзїгУЪЧ____________ЁЃ
(2)ШєАБЦјзуСПЃЌзАжУBжаГіЯжЕФЯжЯѓЮЊ____________ЁЃ
II.ЬНОПАБЫЎЕФЛЙдад
ИУаЫШЄаЁзщЭЌбЇЬНОПВЛЭЌЬѕМўЯТИпУЬЫсМиШмвКгыАБЫЎЕФЗДгІЃЌЪЕбщШчЯТ:
ЪЕбщ | Вйзї | ЯжЯѓ |
Ђй | ШЁ2mL.0.01mol/LKMnO4ШмвКгкЪдЙмжаЃЌМгШыаТПЊЗт1mLХЈАБЫЎЃЌМгШыАыЕЮЙмеєСѓЫЎЃЌеёЕДЃЌгУЯ№ЦЄШћШћзЁЁЃ | ВњЩњзиКжЩЋЮяжЪ(MnO2),дМ10minКѓШмвКзЯКьЩЋБфЧГ |
Ђк | ШЁ2mL0.01mol/LKMnO4ШмвКгкЪдЙмжаЃЌМгШыаТПЊЗт1mLХЈАБЫЎЃЌМгШыАыЕЮЙм1:5ЕФСђЫсЃЌеёЕДЃЌгУЯ№ЦЄШћШћзЁЁЃ | ВњЩњзиКжЩЋЮяжЪ(MnO2),ШмвКзЯКьЩЋСЂПЬБфЧГЃЌдМ2minКѓШмвКзЯКьЩЋЭъШЋЭЫШЅ |
Ђл | ШЁ2mL0.1mol/LKMnO4ШмвКгкЪдЙмжаЃЌМгШыаТПЊЗтImLХЈАБЫЎЃЌМгШыАыЕЮЙмеєСѓЫЎЃЌеёЕДЃЌгУЯ№ЦЄШћШћзЁЁЃ | ВњЩњзиКжЩЋЮяжЪ(MnO2),дМ10minКѓШмвКзЯКьЩЋБфЧГ |
Ђм | ШЁ2mL0.1mol/LKMnO4ШмвКгкЪдЙмжаЃЌМгШыаТПЊЗт1mLХЈАБЫЎЃЌМгШЫАыЕЮЙм1:5ЕФСђЫсЃЌеёЕДЃЌгУЯ№ЦЄШћШћзЁЁЃ | ВњЩњзиКжЩЋЮяжЪ(MnO2)ЃЌШмвКзЯКьЩЋСЂПЬБфЧГЃЌдМ5minКѓШмвКзЯКьЩЋЭъШЋЭЫШЅ |
ЃЈ3ЃЉЪЕбщЂйжабѕЛЏВњЮяЮЊN2ЃЌаДГіИУЗДгІЕФРызгЗНГЬЪН:_________ЁЃ
ЃЈ4ЃЉЪЕбщЂйЂкЫЕУї________________ЁЃ
ЃЈ5ЃЉЪЕбщЂкБШЪЕбщЂмЗДгІЫйТЪ_____(ЬюЁАПьЁАЛђЁАТ§ЁБ)ЃЌдвђЪЧ_________ЁЃ
ЃЈ6ЃЉ1:5ЕФСђЫсШмвК(УмЖШЮЊІб2gЁЄcm-3)ЃЌПЩгУжЪСПЗжЪ§ЮЊ98%ЕФХЈСђЫс(УмЖШЮЊІб1gЁЄcm-3)КЭ
еєСѓЫЎАДЬхЛ§БШ1:5ХфГЩЃЌдђИУ1:5ЕФСђЫсШмвКЕФЮяжЪЕФСПХЈЖШЮЊ_____mol/LЁЃ(гУКЌІб1ЁЂІб2ЕФЪНзгБэЪО)
ЃЈ7ЃЉгЩЪЕбщIЁЂIIПЩЕУГіЕФНсТлЪЧ____________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаРызгМьбщЕФЗНЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
A. ЯђФГШмвКжаМгШыЯѕЫсвјШмвКЃЌЩњГЩАзЩЋГСЕэЃЌЫЕУїдШмвКжагаClЃ
B. ЯђФГШмвКжаМгШыТШЛЏБЕШмвКЃЌЩњГЩАзЩЋГСЕэЃЌЫЕУїдШмвКжагаSO![]()
C. ЯђФГШмвКжаМгШыЧтбѕЛЏФЦШмвКЃЌЩњГЩРЖЩЋГСЕэЃЌЫЕУїдШмвКжагаCu2ЃЋ
D. ЯђФГШмвКжаМгШыТШЛЏБЕШмвКЃЌЩњГЩАзЩЋГСЕэЃЌдйМгбЮЫсГСЕэВЛШмНтЃЌЫЕУїдШмвКжагаSO![]()
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com