
����Ŀ����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ���ֳ�Ϊ���ȼҵ��������������Ϊԭ������һϵ�л�����Ʒ��Ϊ���ԭ�ϵ������ʣ����ܽ��ġ������ͼ��ʾ�������̣������ȼҵװ���еĵ缫δ�����
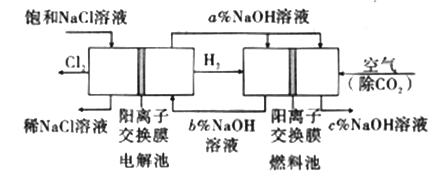
(1)��ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ___________________��
(2)Ϊ��ȥ�����е�Ca2+��Mg2+��SO42-����ɳ���õ�������NaCl���ɽ���������ˮ����ȷ�IJ��������˳����_______ (����ţ���
�ٹ��ˢڼӹ���NaOH��Һ�ۼ���������ܼӹ���Na2CO3��Һ�ݼӹ���BaCl2��Һ
A.�٢ܢ٢ڢݢ� B.�٢ڢݢܢ٢� C.�٢ڢܢݢ� D.�ܢڢ�
(3)ͼ��NaOH��Һ����������a%��b%��c%���ɴ�С��˳��Ϊ_________��
(4)�ȼҵ�IJ���NaOH�벻ͬ���ʷ�Ӧ�������ɲ�ͬ���Ρ���֪�����£�Ũ�Ⱦ�Ϊ0.1 mol/L������������ҺpH���±�������˵������ȷ����_______(�����)��
���� | Na2CO3 | NaHCO3 | NaClO | NaHSO3 |
pH | 11.6 | 9.7 | 10.3 | 5.2 |
A.����ˮ�м���NaHCO3������������ˮ�д������Ũ��
B.������Һ�У�ˮ�ĵ���̶�������NaClO
C.�����£���ͬ���ʵ���Ũ�ȵ�H2SO3��H2CO3��HClO��pH������HClO
D.�����ε������ӽ��H+������ǿ����HCO3-
(5)�����õ���Ȼ�����Һ���õ�������36.5%��Ũ����100t��������Ҫ����ʳ��_________t��
(6)�ȼҵ��ƷCl2������ұ��������õ������ѣ�������ͼ��д���������Ȼ����õ����Ȼ��ѵĻ�ѧ����ʽ��____________________��
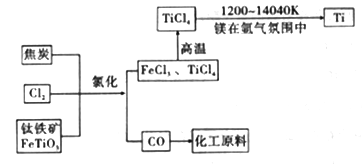
(7)��������������ֻ�ѧ��������ȶ������塣þ��ԭTiCl4�ķ�Ӧ��Ϊ��ֹMg��Ti������ѡ��ϡ��������������û�ѧ��Ӧ����ʽ���Ͳ�ѡ�õ�����ԭ��__________________��
���𰸡� 2NaCl��2H2O![]() 2NaOH��H2����Cl2�� B c%>a%>b% BD 58.5 2FeTiO3+6C+7Cl2=2FeCl3+2TiCl4+6CO 3Mg +N2
2NaOH��H2����Cl2�� B c%>a%>b% BD 58.5 2FeTiO3+6C+7Cl2=2FeCl3+2TiCl4+6CO 3Mg +N2![]() Mg3N2
Mg3N2
�����������⿼�黯ѧ�������̣���1����ⱥ��ʳ��ˮ�Ļ�ѧ��Ӧ����ʽΪ2NaCl��2H2O![]() 2NaOH��H2����Cl2������2����ȥCa2����Na2CO3����ȥMg2����NaOH����ȥSO42����BaCl2�����Ӳ������������ʣ���̼���ƻ��г�ȥ������BaCl2�����ã���Na2CO3����BaCl2�ĺ��棬���˳����NaOH��BaCl2��Na2CO3����BaCl2��NaOH��Na2CO3����BaCl2��Na2CO3��NaOH��Ȼ����ˣ��ټ������ᣬ��ѡ��B��ȷ����3������������һ�����缫��ӦʽΪ2H2O��2e��=H2����2OH�������a%>b%��ͨ����һ������O2��2H2O��4e��=4OH���������c%>a%,�����c%>a%>b%����4����������ˮ��Ĺ��ɣ�Խ��Խˮ�⣬�������H��������H2SO3>H2CO3>HClO>HCO3����A����ˮ�д���Cl2��H2O
2NaOH��H2����Cl2������2����ȥCa2����Na2CO3����ȥMg2����NaOH����ȥSO42����BaCl2�����Ӳ������������ʣ���̼���ƻ��г�ȥ������BaCl2�����ã���Na2CO3����BaCl2�ĺ��棬���˳����NaOH��BaCl2��Na2CO3����BaCl2��NaOH��Na2CO3����BaCl2��Na2CO3��NaOH��Ȼ����ˣ��ټ������ᣬ��ѡ��B��ȷ����3������������һ�����缫��ӦʽΪ2H2O��2e��=H2����2OH�������a%>b%��ͨ����һ������O2��2H2O��4e��=4OH���������c%>a%,�����c%>a%>b%����4����������ˮ��Ĺ��ɣ�Խ��Խˮ�⣬�������H��������H2SO3>H2CO3>HClO>HCO3����A����ˮ�д���Cl2��H2O![]() HCl��HClO������NaHCO3������NaHCO3��HCl=NaCl��CO2����H2Ŷ,NaHCO3��������ᷴӦ����ʹƽ�����ƣ�HClO��Ũ������A��˵����ȷ��B���Ա�pH��pH���˵��ˮ��̶ȴ�Na2CO3ˮ��̶����B˵������C����������������HClO������������pH���C��˵����ȷ��D���������������������H������Խǿ����Ӧ��������H������Խ������CO32����ǿ����D˵������5��Ũ������HCl�����ʵ���Ϊ100��106��36.5%/36.5mol=106mol��������Ԫ���غ㣬n(NaCl)=n(HCl)=106mol��������Ϊ106��58.5g=58.5��106g����58.5t����6���������̣���Ӧ����ʽΪ2FeTiO3��6C��7Cl2=2FeCl3��2TiCl4��6CO����7����ΪMg��N2��Ӧ�����ɵ���þ����˷�Ӧ����Ϊ3Mg +N2
HCl��HClO������NaHCO3������NaHCO3��HCl=NaCl��CO2����H2Ŷ,NaHCO3��������ᷴӦ����ʹƽ�����ƣ�HClO��Ũ������A��˵����ȷ��B���Ա�pH��pH���˵��ˮ��̶ȴ�Na2CO3ˮ��̶����B˵������C����������������HClO������������pH���C��˵����ȷ��D���������������������H������Խǿ����Ӧ��������H������Խ������CO32����ǿ����D˵������5��Ũ������HCl�����ʵ���Ϊ100��106��36.5%/36.5mol=106mol��������Ԫ���غ㣬n(NaCl)=n(HCl)=106mol��������Ϊ106��58.5g=58.5��106g����58.5t����6���������̣���Ӧ����ʽΪ2FeTiO3��6C��7Cl2=2FeCl3��2TiCl4��6CO����7����ΪMg��N2��Ӧ�����ɵ���þ����˷�Ӧ����Ϊ3Mg +N2![]() Mg3N2��
Mg3N2��


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ���ǣ� ��
A.Ħ���ǹ��ʵ�λ�����߸�����������֮һ
B.���³�ѹ�£�11.2LCO2����������Ϊ0.5NA
C.H2SO4 ��Ħ������Ϊ98 g
D.6.02��1022��H2SO4���ӵ�����Ϊ9.8 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���
ѡ�� | �� | �� | �� | ʵ����� |
|
A | Ũ��ˮ | NaBr | ����KI��Һ | �����ԣ�Cl2>Br2>I2 | |
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | Br2�ı���Һ | ��м | AgNO3��Һ | �����嵥���������������� ����ȡ����Ӧ | |
D | ���� | Na2SO3 | KMnO4��Һ | SO2��ʹKMnO4��Һ��ɫ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�����������һ��ֲ���к��п������õĻ�����M����֪M�ĺϳ�·������ͼ��ʾ:
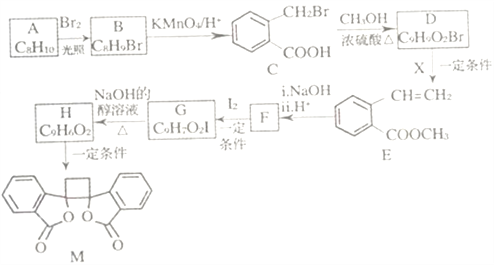
��֪:i.RCH2Br![]() R-HC=CH-R'��
R-HC=CH-R'��
ii.R-HC=CH-R'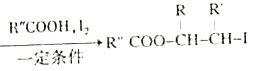 ��
��
iii.R-HC=CH-R'![]()
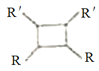 (����R��R'��R"�����⡢������)��
(����R��R'��R"�����⡢������)��
(1)A�Ļ�ѧ����Ϊ_______��D�����������ŵ�������______����H����M�ķ�Ӧ����Ϊ_____��
(2)�����Ƚ��ķ�������������������л���ṹ������
�ٿ�����_______(����ס���������ס��˴Ź�������)���C����Է���������
�ڲⶨB�Ľṹ:B�ĺ˴Ź���������ʾΪ_____��塣
(3)1molM������NaOH��Һ��Ӧ������_____molNaOH��
(4)X�ĽṹʽΪ_____��F�Ľṹ��ʽΪ______ ��
(5)��C����H�Ļ�ѧ����ʽΪ__________��
(6)��������������B��ͬ���칹�干______��(�����������칹)��
�ٷ����廯���� ����E������ͬ������ ���ܷ���������Ӧ ������������ˮ��������Ȼ�����Һ����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڲ�ͬ�¶��£�ˮ��Һ��c(H��)��c(OH��)����ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ����( )
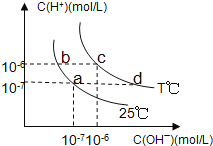
A. a���Ӧ����Һ�д������ڣ�Fe3+��Na+��Cl�D��SO42�D
B. b���Ӧ����Һ�д������ڣ�NH4+��Ba2+��OH�D��I�D
C. c���Ӧ����Һ�д������ڣ�Na+��Ba2+��Cl�D��HCO3�D
D. d���Ӧ����Һ�д������ڣ�Na+��K+��SO32�D��Cl�D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ijͬѧ�����һ�����ѣ�CH3OCH3��ȼ�ϵ�ز�̽���ȼҵԭ����ԭ���ʹ�ͭ�ľ���ԭ������װ����XΪ�����ӽ���Ĥ������Ҫ��ش�����������⣺
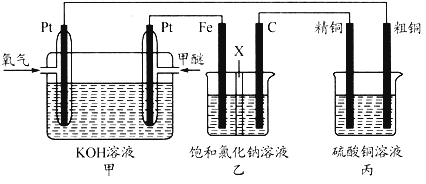
��1��ͨ�������ĵ缫Ϊ_____(��������������������)��д�������ĵ缫��Ӧʽ_____��
��2�����缫Ϊ_____(��������������������)��ʯī�缫�ĵ缫��ӦʽΪ_____��
��3����Ӧһ��ʱ�����װ��������NaOH��Ҫ��_____(��������������ʯī����)����
��4�������ͭ�к���п���������ʣ���װ���������ϵ缫��ӦʽΪ_____����Ӧһ��ʱ�䣬����ͭ��ҺŨ�Ƚ�_____(��������������С������������)��
��5�����ڱ�״���£���2.24L�����μӷ�Ӧ������װ�������缫�����ɵ������ڱ�״���µ����Ϊ_____����װ������������ͭ������Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˼���ij��Һ���Ƿ��г��������������ӣ�ij��ѧС���ͬѧ������������ʾ��ʵ����������м�������в�����������ʹʪ��ĺ�ɫʯ����ֽ�������ɸ�ʵ���ܵó�����ȷ�����ǣ� ��

A��ԭ��Һ��һ������SO42- B��ԭ��Һ��һ������NH4+
C��ԭ��Һ��һ������Cl- D��ԭ��Һ��һ������Fe3+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ǰ������Ԫ��A��B��C��D��E��F��ԭ������������������A��Bͬ���ڣ���̬��AB2��������C��Eԭ�Ӷ���һ��δ�ɶԵ��ӣ�C+��E-��һ�����Ӳ㣬Eԭ�ӵõ�һ�����Ӻ�3p���ȫ������D�����������D����������Ϊ40%���Һ���������������������FΪ��ɫ���ʣ���F+��F2+�������ӡ��ش��������⣺
��1��Ԫ�ص縺�ԣ�D____E (�������)��
��2��B��C�����۵�B_____C(�������)��
��3��AE4��Aԭ���ӻ������ʽΪ��________�ӻ������̬��������Ϊ_____________��
��4���⻯��ķе㣺B��D�ߵ�ԭ��______________��
��5��F�ĺ�������Ų�ʽΪ____________________________����F������������μ��백ˮ�Ȳ���������������ܽ�Ϊ����ɫ��Һ�������Ҵ�����������ɫ���壬�þ���Ļ�ѧʽΪ_______�����й��ڸþ�������˵������ȷ����_____________________��
A�������Ҵ���Ŀ���ǽ����ܼ��ļ��ԣ���ʹ��������
B��F��NH3֮��Ļ�ѧ��Ϊ���Ӽ�
C��������ᄃ���У�N����λԭ�ӣ�NH3Ϊ������
D����������Nԭ�����г�Ϊƽ�������Σ�������F������sp3�ӻ�
E����þ����ˮ��Һ�м���ŨBaCl2��Һ�а�ɫ��������
��6��Ԫ��X��ij��̬������Xn-�����е������ó���K��L���Ӳ㣬CnX�������С�ṹ��ԪΪ�����壬�ṹ��ͼ��ʾ���þ�����ÿ��Xn-��________���Ⱦ����C+���Ӱ�Χ����֪�þ�����ܶ�Ϊ��g��cm-3�������ӵ�����ΪNA��CnX��Ħ������ΪM g/mol��C+��Xn-�����̾�����_____________nm�����г�����ʽ���ɣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ч�ļ���ȼ�ϵ�ز��ò�Ϊ�缫���ϣ����缫�Ϸֱ�ͨ��CH4��O2 �������ΪKOH��Һ��ij�о�С�齫��������ȼ�ϵ�ش�������Ϊ��Դ�����б����Ȼ�����Һ���ʵ�飬��ͼ��ʾ��

�ش��������⣺
��1������ȼ�ϵ�������������ĵ缫��Ӧ�ֱ�Ϊ________________ ��_______________ ��
��2���պ�K���غ�a��b�缫�Ͼ����������������a�缫�ϵõ�����_________���ѧʽ��������Ȼ�����Һ���ܷ�Ӧ����ʽΪ___________________��
��3����ÿ����ؼ���ͨ����Ϊ1 L(��״��)���ҷ�Ӧ��ȫ��������������ܲ������������Ϊ____________L(��״��)��
��4���������Ȼ�����Һ���Ϊ100mL�����һ��ʱ������²����ҺpHΪ13����Ҫʹ��Һ�ָ������ǰ��״̬��������Һ�мӣ���ͨ����__________����д���ʵĻ�ѧʽ��_________g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com