
����Ŀ��ʵ��������ͭм�����������Ļ���Ϊԭ���Ʊ�����ͭ���塣��Ͼ���������̻ش��������⡣
(1)���ƻ����3 mol/L�����ᣨ�ܶ�1.180g/cm3����15mol/L��Ũ���ᣨ�ܶ�1.400 g/cm3���������5��1��Ϻ���ȴ��
�ټ���������������������Ϊ__________��
��ȡ1g���ᣬ��ˮϡ����20.00mL����0.5mol/L�ռ���еζ������ı��ռ���Һ�����Ϊ__________mL��
(2)���շ�ͭм������һ���������溬���۵Ĵ�ͭм��ͭ����Ϊ99.84%�����������������գ������۳���������ȥ��ֱ��ͭм������ʺ�ɫ����ȴ���������������������ǰ������3.2 %��
�ٹ�������Ԫ�ص���������Ϊ__________������3λС������
�ڹ�����ͭ������ͭ�����ʵ���֮��Ϊ___________��
(3)�ܽ⣺��ȡ2.064g���壬������������һ�������Ļ��ᣬǡ����ȫ��Ӧ����ʽ�������NO��������ڱ�״���µ������������Ļ�ԭ����ֻ��NO����______________
(4)�ᾧ������Ӧ�����Һˮԡ����Ũ������ȴ�ᾧ�������������塣
�ټ��㷴Ӧ����Һ��CuSO4�����ʵ�����__________��
�������յõ�������������Ϊ6.400g�������IJ���Ϊ_________������ȷ��1%��
���𰸡�0.20 12.33 0.033 45��7 403.2 0.0268mol 82%
��������
(1)��������������������������ʵ��������Ի����Һ��������
�������ӵ����ʵ����������������ʵ�����ȣ�
(2)����������ΪOԪ�أ�
��OԪ�ص����ʵ�����������ͭ�����ʵ�����
(3)103.2g����ﺬͭ1.35mol�����ݵ��ӵ�ʧ�غ����һ�����������ʵ�����
(4)�ٹ����к�ͭ0.027mol������ͭ0.0042mol�������Ļ�����H2SO4��HNO3�����ʵ�����Ϊamol��ǡ����ȫ��Ӧ����Һ������ΪCuSO4��Cu(NO3)2�����ݵ���غ���⣻
�ڹ����к�ͭ0.027mol������ͭ0.0042mol������ͭԪ���غ�������۲�����
(1)������������������ֱ�Ϊ5mL��1mL������������ʵ���������������ʵ��������������������![]() ���ʴ�Ϊ��0.20��
���ʴ�Ϊ��0.20��
������Ϊ7.3g�Ļ����к�����������ʵ���=![]() �����Ͽ�֪��������ʵ���=
�����Ͽ�֪��������ʵ���=![]() ��������0.045molH+����1g�����к���H+Ϊ
��������0.045molH+����1g�����к���H+Ϊ![]() mol���к�ʱ��ҪNaOH��Һ�������V=
mol���к�ʱ��ҪNaOH��Һ�������V=![]() =12.33mL���ʴ�Ϊ��12.33��
=12.33mL���ʴ�Ϊ��12.33��
(2)����ͭм��100g����ͭ99.84g�����պ������Ϊ103.2g������Ԫ�ص�����Ϊ3.36g����Ԫ�ص���������Ϊ![]() ���ʴ�Ϊ��0.033��
���ʴ�Ϊ��0.033��
���������Ϊ103.2g��������ͭ�����ʵ���=��Ԫ�ص����ʵ���=![]() ��ͭ�����ʵ���Ϊ
��ͭ�����ʵ���Ϊ![]() ��ͭ������ͭ�����ʵ���֮��Ϊ1.35mol��0.21mol=45:7���ʴ�Ϊ��45:7��
��ͭ������ͭ�����ʵ���֮��Ϊ1.35mol��0.21mol=45:7���ʴ�Ϊ��45:7��
(3)103.2g����ﺬͭ1.35mol��2.064g�����к�ͭ0.027mol�����ݵ��ӵ�ʧ�غ��֪һ�����������ʵ���Ϊ0.027mol��2/3=0.018mol����״���µ����Ϊ0.018mol��22.4L/mol��1000mL/L=403.2mL���ʴ�Ϊ��403.2mL��
(4)�ٹ����к�ͭ0.027mol������ͭ0.0042mol�������Ļ�����H2SO4��HNO3�����ʵ�����Ϊamol��ǡ����ȫ��Ӧ����Һ������ΪCuSO4��Cu(NO3)2�����ݵ���غ����������������¹�ϵʽ��2��(0.027mol+0.0042mol)=2a+a0.018mol�����a=0.0268mol����Һ��CuSO4�����ʵ���Ϊ0.0268mol���ʴ�Ϊ��0.0268mol��
�ڵ��������۲���Ϊ(0.027+0.0042)mol��250g/mol=7.8g������Ϊ![]() ���ʴ�Ϊ��82%��
���ʴ�Ϊ��82%��


 �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��WΪԭ�����������Ķ���������Ԫ�أ�Y�ļ���̬�⻯��ˮ��Һ�������ԣ� ZԪ�������ۣ��һ�̬ԭ����2��δ�ɶԵ��ӣ���̬Wԭ�Ӽ۲�����Ų�ʽΪnsn-1npn-1 ��X��WΪͬ����Ԫ�ء���̬Rԭ��M�ܲ�ȫ�����Һ������ҽ���1��δ�ɶԵ��ӡ���ش��������⣺
��1����̬Rԭ�ӵĺ�������Ų�ʽΪ____________________��R���ʾ��徧���Ķѻ���ʽ_______________�������Ŀռ�������Ϊ___________________��
��2�� X��Y��Z����Ԫ�صĵ�һ��������С�����˳��Ϊ__________ (����Ԫ�ط���������ͬ��
��3��YF3������Y���ӻ�����Ϊ______________���÷��ӵĿռ乹��Ϊ_________________��
��4��Y����̬�⻯����ˮ�п��γ���������������ܵ���ʽΪ___________________��
��5��X��ij��̬���������Է�������Ϊ44�������еĴ��������÷��Ŧ�![]() ��ʾ������m���������γɴ�������ԭ������n���������γɴ������ĵ�������������̬�������еĴ�����Ӧ��ʾΪ_______________����������������Ŀ֮��Ϊ______________��
��ʾ������m���������γɴ�������ԭ������n���������γɴ������ĵ�������������̬�������еĴ�����Ӧ��ʾΪ_______________����������������Ŀ֮��Ϊ______________��
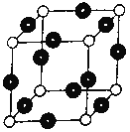
��6��RԪ����YԪ���γ�ij�ֻ�����ľ����ṹ��ͼ��ʾ���������Rԭ�ӣ������þ�����ܶ�Ϊ��g��cm-3����þ����ı߳���_________cm (NA��ʾ����٤��������ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪ��ˮ�ۺ����õIJ������̣������й�˵����ȷ����
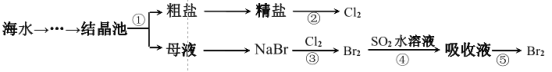
A.ʵ���ҽ��в������Ҫ������������������
B.�������һ������ѧ��ת��Ϊ���ܵĹ���
C.����ۡ����漰�Ļ�ѧ��Ӧ��Ϊ������ԭ��Ӧ
D.������е����ӷ���ʽΪ SO2��Br2��2H2O=4H����SO32-��2Br-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ���������ֵ�������й�������ȷ���ǣ� ��
A.2.24LCO2������Na2O2��Ӧ��ת�Ƶĵ�����ĿΪ0.1NA
B.1molCH4��������C12�ڹ����·�Ӧ���ɵ�CH3C1������ΪNA
C.��֪��Ӧ2SO2(g)+O2(g)![]() 2SO3(g) ��H=-akJ/mol��������NA��SO3����ʱ����Ӧ�ų�������С��0.5akJ
2SO3(g) ��H=-akJ/mol��������NA��SO3����ʱ����Ӧ�ų�������С��0.5akJ
D.��1L0.1mol/L̼������Һ�У���������������0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й��Ȼ�ѧ����ʽ��������ȷ���ǣ� ��
A.2H2(g)+O2(g)=2H2O(g) ��H=+483.6kJ/mol
B.��֪C(ʯī��s)=C(���ʯ��s) ��H>0������ʯ��ʯī�ȶ�
C.CO(g)��ȼ���ȡ�H=-283kJ/mol����2CO2(g)=2CO(g)+O2(g)��H=+566kJ/mol
D.��֪2C(s)+2O2(g)=2CO2(g) ��H1��2C(s)+O2(g)=2CO(g) ��H2������H1>��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ڹ��ý�������������ҪӦ�ã�þ����������ͭ�Ǽ�����Ҫ�Ľ���Ԫ�ء���ش��������⣺
��1����Ԫ�صĺ˵����Ϊ28����ԭ�ӻ�̬�����Ų�ʽΪ__���ṹ����__�ֲ�ͬ��״�ĵ����ơ�
��2��þ����Ԫ�����ڱ��е�__��Ԫ�أ�MgO���۵����CuO��ԭ����__��
��3���Ȼ�������CrO2Cl2���۵㣺-96.5�棬�е㣺117�棬����CS2�Ȼ��ܣ����̬CrO2Cl2����__���塣��֪NO2+��CS2��Ϊ�ȵ����壬��1molNO2+�к���������ĿΪ__��
��4�������[Cu(CH3C��N)4]BF4��̼ԭ���ӻ��������Ϊ__��[Cu(CH3C��N)4]+������Ԫ�صĵ縺���ɴ�С��˳����__��BF4-�Ŀռ乹��Ϊ__��
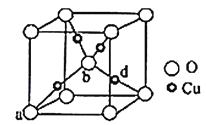
��5��ͭ����Ԫ�ؿ��γ���ͼ��ʾ�ľ����ṹ������Cu���ȵط�ɢ���������ڲ���a��b�������������Ϊ��0��0��0������![]() ��
��![]() ��
��![]() ������d���������Ϊ__����֪��Ʒ����ܶ�Ϊ��g/cm3��NA�ǰ����ӵ�����ֵ���������������ⳤ��Ϊ__cm���г�����ʽ���ɣ���
������d���������Ϊ__����֪��Ʒ����ܶ�Ϊ��g/cm3��NA�ǰ����ӵ�����ֵ���������������ⳤ��Ϊ__cm���г�����ʽ���ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2A+B![]() 3C+4D��Ӧ�У���ʾ�÷�Ӧ���������ǣ� ��
3C+4D��Ӧ�У���ʾ�÷�Ӧ���������ǣ� ��
A.v��A��=0.4mol/��L��s��
B.v��B��=0.3mol/��L��s��
C.v��C��=0.3mol/��L��s��
D.v��D��=2mol/��L��min��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ����������������ԭ�����͵��ǣ� ��
A.��������ˮ�е�NO2����ɫ��dz
B.�ϳɰ���ҵ�в��ϴӷ�Ӧ�������Һ�����������
C.ʵ�����г����ű���ʳ��ˮ�ķ����ռ�Cl2
D.ʹ�ô���ʹ�ϳɰ����ʼӿ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(ClO2)����ǿ�����ԣ��ڹ�ҵ�ϳ�����ˮ��������Ư����ClO2��һ��������ˮ�Ļ���ɫ���壬�������������10%ʱ������ը��ij�о�С�������������ַ����Ʊ�ClO2���ش��������⣺
��1���Ի�����(FeS2)�������ƺ�������Һ��Ϸ�Ӧ�Ʊ�ClO2���������е���Ԫ�������������±�ClO3-������SO42-��д���Ʊ�ClO2�����ӷ���ʽ__��
��2���ù�����������ԭ��������������л�ԭNaClO3�Ʊ�ClO2�������Ƶõ�ClO2���ڴ�����CN-��ˮ��ʵ����ģ��ù��̵�ʵ��װ��(�г�װ����)��ͼ��ʾ��
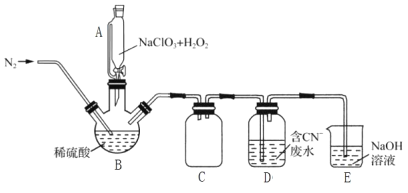
��װ��A��������__��װ��C��������__��
�ڷ�Ӧ����BӦ����30�����ҵ�ˮԡ�У�Ŀ����__��
��ͨ�뵪������Ҫ������3����һ�ǿ����������ã����������ڽ�ClO2�ų�������__��
��ClO2������CN-��ˮ�����ӷ���ʽΪ__��װ��E��������__��
��3���Ȼ��Ƶ�ⷨ��һ�ֿɿ��Ĺ�ҵ����ClO2�ķ�����
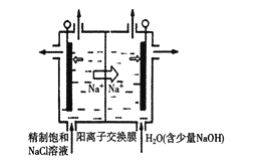
�����ڵ���ʳ��ˮ���ȳ�ȥ���е�Ca2+��Mg2+��SO42-�����ʡ�ij�γ��Ӳ���ʱ��������ˮ���ȼ��������__���ѧʽ�������������ٲ������ټ��������Na2CO3��NaOH����ַ�Ӧ����һ����ȥ��
����ʯī���缫����һ�������µ�ⱥ��ʳ��ˮ��ȡClO2������ԭ����ͼ��ʾ��д����������ClO2�ĵ缫��Ӧʽ__��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com