
ЁОЬтФПЁПРћгУЯТЭМзАжУВтЖЈжаКЭШШЕФЪЕбщВНжшШчЯТЃК

ЂйгУСПЭВСПШЁ50 mL 0.25 mol/LСђЫсЕЙШыаЁЩеБжаЃЌВтГіСђЫсШмвКЮТЖШЃЛ
ЂкгУСэвЛСПЭВСПШЁ50 mL 0.55 mol/L NaOHШмвКЃЌВЂВтГіЦфЮТЖШЃЛ
ЂлНЋNaOHШмвКЕЙШыаЁЩеБжаЃЌЩшЗЈЪЙжЎЛьКЯОљдШЃЌВтГіЛьКЯвКзюИпЮТЖШЁЃ
ЛиД№ЯТСаЮЪЬтЃК
(1)ЕЙШыNaOHШмвКЕФе§ШЗВйзїЪЧ_________
AЃЎбиВЃСЇАєЛКТ§ЕЙШы BЃЎЗжШ§ДЮЩйСПЕЙШы CЃЎвЛДЮбИЫйЕЙШы
(2)ЪЙСђЫсгыNaOHШмвКЛьКЯОљдШЕФе§ШЗВйзїЪЧ__________
AЃЎгУЮТЖШМЦаЁаФНСАш BЃЎНвПЊгВжНЦЌгУВЃСЇАєНСАш CЃЎЧсЧсЕиеёЕДЩеБ DЃЎгУЬздкЮТЖШМЦЩЯЕФЛЗаЮВЃСЇАєЩЯЯТЧсЧсЕиГщЖЏ
(3)ЪЕбщЪ§ОнШчЯТБэЃК
ЮТЖШ ЪЕбщДЮЪ§ЁЁ | Ц№ЪМЮТЖШt1Ёц | жежЙЮТЖШt2/Ёц | ЮТЖШВюЦНОљжЕ (t2Ѓt1)/Ёц | ||
H2SO4 | NaOH | ЦНОљжЕ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
ЂйИљОнБэжаЪ§ОнМЦЫуГіРДЮТЖШВюЕФЦНОљжЕЮЊ______ЁцЃЛ
ЂкНќЫЦШЯЮЊ0.55 mol/L NaOHШмвККЭ0.25 mol/LСђЫсШмвКЕФУмЖШЖМЪЧ1 g/cm3ЃЌжаКЭКѓЩњГЩШмвКЕФБШШШШнcЃН4.18 J/(gЁЄЁц)ЁЃдђжаКЭШШІЄHЃН_______( ШЁаЁЪ§ЕуКѓвЛЮЛ)ЁЃ
ЂлЩЯЪіЪЕбщЪ§жЕНсЙћгыжаКЭШШЮЊ57.3 kJ/molгаЦЋВюЃЌВњЩњЦЋВюЕФдвђПЩФмЪЧ____ЁЃ
aЃЎЪЕбщзАжУБЃЮТЁЂИєШШаЇЙћВю bЃЎгУЮТЖШМЦВтЖЈNaOHШмвКЦ№ЪМЮТЖШКѓжБНгВтЖЈH2SO4ШмвКЕФЮТЖШ cЃЎСПШЁNaOHШмвКЕФЬхЛ§ЪБбіЪгЖСЪ§ dЃЎЗжЖрДЮАбNaOHШмвКЕЙШыЪЂгаСђЫсЕФаЁЩеБжа
ЁОД№АИЁПC D 3.4 -56.8kJ/mol abcd
ЁОНтЮіЁП
БОЪЕбщЕФФПЕФЪЧВтЖЈжаКЭШШЃЌжаКЭШШЪЧжИЪЧЧПЫсКЭЧПМюЕФЯЁШмвКЭъШЋЗДгІЩњГЩ1molЫЎЗХГіЕФШШСПЃЛБОЪЕбщжаЪзЯШВтЖЈЫсКЭМюЗДгІЧАЕФЮТЖШЃЌШЛКѓВтЖЈЗДгІжежЙЮТЖШЃЌШЛКѓРћгУБШШШШнНЋЮТЖШзЊЛЏЮЊШШСПМЦЫуГіжаКЭШШЁЃБОЪЕбщжаЮЊБЃжЄЫсКЭМюЭъШЋЗДгІЃЌNaOHЙ§СПЃЛЪЕбщЕФЙиМќЪЧвЊБЃЮТЁЃ
(1)ЮЊСЫМѕЩйШШСПЕФЩЂЪЇЃЌЪЕбщЙ§ГЬжаЕЙШыNaOHШмвКЪБЃЌБиаывЛДЮбИЫйЕФЕЙШыЃЌЫљвдбЁCЃЛ
(2)ЮТЖШМЦЪЧВтСПЮТЖШЕФЃЌВЛФмЪЙгУЮТЖШМЦНСАшЃЛвВВЛФмЧсЧсЕиеёЕДЩеБЃЌЗёдђПЩФмЕМжТвКЬхНІГіЛђШШСПЩЂЪЇЃЌгАЯьВтЖЈНсЙћЃЛИќВЛФмДђПЊгВжНЦЌгУВЃСЇАєНСАшЃЌЗёдђЛсгаШШСПЩЂЪЇЃЛЪЙСђЫсгыNaOHШмвКЛьКЯОљдШЕФе§ШЗВйзїЗНЗЈЪЧЃКгУЬздкЮТЖШМЦЩЯЕФЛЗаЮВЃСЇНСАшАєЧсЧсЕиНСЖЏЃЌЫљвдбЁDЃЛ
(3)ЂйЫФДЮЕФЮТВюЗжБ№ЮЊ3.4ЁцЃЌ5.1ЃЌ3.3ЁцЃЌ3.5ЁцЃЌЕк2зщЪ§ОнЦЋВюНЯДѓЩсШЅЃЌЫљвдЦНОљЮТВюЮЊ![]() =3.4ЁцЃЛ
=3.4ЁцЃЛ
Ђк50mL0.25mol/LСђЫсгы50mL0.55mol/LNaOHШмвКНјаажаКЭЗДгІЃЌNaOHЙ§СПЃЌЫљвдЩњГЩЫЎЕФЮяжЪЕФСПЮЊ0.05LЁС0.25mol/LЁС2=0.025molЃЌШмвКЕФжЪСПЮЊ100mlЁС1g/cm3=100gЃЌЮТЖШБфЛЏЕФжЕЁїT=3.4ЁцЃЌдђЩњГЩ0.025molЫЎЗХГіЕФШШСПЮЊQ=mcЁїT=100gЁС4.18J/(gЁц)ЁС3.4Ёц=1421.2JЃЌМД1.4212kJЃЌЫљвдЪЕбщВтЕУЕФжаКЭШШЁїH=-![]() =-56.8kJ/molЃЛ
=-56.8kJ/molЃЛ
ЂлЪЕбщНсЙћЕФОјЖджЕаЁгкжаКЭШШЕФОјЖджЕЃЛ
aЃЎЪЕбщзАжУБЃЮТЁЂИєШШаЇЙћВюЃЌШШСПЩЂЪЇНЯДѓЃЌЫљЕУжаКЭШШЕФОјЖджЕЦЋаЁЃЌЙЪaЗћКЯЃЛ
bЃЎгУЮТЖШМЦВтЖЈNaOHШмвКЦ№ЪМЮТЖШКѓжБНгВтЖЈH2SO4ШмвКЕФЮТЖШЃЌЮТЖШМЦЩЯеДгаЕФNaOHгыСђЫсЗДгІЗХГіШШСПЃЌЪЙЕУВтЖЈЕФГѕЪМЮТЖШЦЋИпЃЌЧвдьГЩШШСПЕФЩЂЪЇЃЌВтЖЈЕФЮТВюЦЋаЁЃЌжаКЭШШЕФОјЖджЕЦЋаЁЃЌЙЪbЗћКЯЁЃ
cЃЎСПШЁNaOHШмвКЕФЬхЛ§ЪБбіЪгЖСЪ§ЃЌЛсЕМжТЧтбѕЛЏФЦЬхЛ§ЦЋДѓЃЌЕЋСђЫсЩйСПЃЌЫљвдЩњГЩЕФЫЎЕФЮяжЪЕФСПВЛБфЃЌЗХГіЕФзмШШСПВЛБфЃЌЖјМгШыЕФNaOHШмвКЬхЛ§ЦЋДѓЃЌЛсЪЙЛьКЯвКЕФжЪСПЦЋДѓЃЌдђВтЕУЕФЮТВюЛсЦЋаЁЃЌжаКЭШШЕФОјЖджЕЦЋаЁЃЌЙЪcЗћКЯЃЛ
dЃЎЗжЖрДЮАбNaOHШмвКЕЙШыЪЂгаСђЫсЕФаЁЩеБжаЃЌШШСПЩЂЪЇНЯДѓЃЌЫљЕУжаКЭШШЕФОјЖджЕЦЋаЁЃЌЙЪdЗћКЯЃЛ
ЙЪД№АИЮЊЃКabcdЃЛ


 аТПЮБъНзЬндФЖСбЕСЗЯЕСаД№АИ
аТПЮБъНзЬндФЖСбЕСЗЯЕСаД№АИ ПкЫуаФЫуЫйЫугІгУЬтЯЕСаД№АИ
ПкЫуаФЫуЫйЫугІгУЬтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГгаЛњЮяЕФНсЙЙМђЪНШчЯТЃЌЯТСагаЙиИУгаЛњЮяЕФа№ЪіжаВЛе§ШЗЕФЪЧ
![]()
A. ЦфЗжзгЪНЮЊ C9H10O
B. ФмЪЙЫсадИпУЬЫсМиШмвКЭЪЩЋ
C. вЛЖЈЬѕМўЯТЃЌ1mol ИУгаЛњЮяРэТлЩЯзюЖрФмгы 4mol H2 ЗЂЩњМгГЩЗДгІ
D. вЛЖЈЬѕМўЯТЃЌ1mol ИУгаЛњЮяРэТлЩЯзюЖрФмгы 4mol Br2 ЗЂЩњМгГЩЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЭЌбЇНјааSO2ЕФаджЪЪЕбщЁЃдкЕуЕЮАхaЁЂbЁЂcЁЂdДІЗжБ№ЕЮгаВЛЭЌЕФЪдМСЃЌдйЯђNa2SO3ЙЬЬхЩЯЕЮМгЪ§ЕЮХЈH2SO4КѓЃЌдкећИіЕуЕЮАхЩЯИЧЩЯХрбјУѓЃЌвЛЖЮЪБМфКѓЙлВьЕНЕФЪЕбщЯжЯѓШчБэЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
ађКХ | ЪдМС | ЪЕбщЯжЯѓ |
a | ЦЗКьШмвК | КьЩЋЭЪШЅ |
b | ЫсадKMnO4ШмвК | зЯЩЋЭЪШЅ |
c | NaOHШмвКЃЈКЌ2ЕЮЗгЬЊЃЉ | КьЩЋЭЪШЅ |
d | H2SШмвК | ЛЦЩЋЛызЧ |
A.дкХЈСђЫсгыNa2SO3ЙЬЬхЗДгІжаЃЌХЈСђЫсБэЯжЕФЧПбѕЛЏад
B.aЁЂbОљБэУїSO2ОпгаЦЏАзад
C.cжажЛПЩФмЗЂЩњЗДгІЃКSO2+2OH-=SO32-+H2O
D.dжаБэУїSO2ОпгабѕЛЏад
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAs2O3дквНвЉЁЂЕчзгЕШСьгђгаживЊгІгУЁЃФГКЌЩщдЊЫиЃЈAsЃЉЕФЙЄвЕЗЯЫЎОШчЭМ1СїГЬзЊЛЏЮЊДжВњЦЗЁЃ
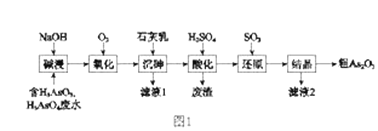
ЃЈ1ЃЉЁАМюНўЁБЕФФПЕФЪЧНЋЗЯЫЎжаЕФH3AsO3КЭH3AsO4зЊЛЏЮЊбЮЁЃH3AsO4зЊЛЏЮЊNa3AsO4ЗДгІЕФЛЏбЇЗНГЬЪНЪЧ_______________________________ЁЃ
ЃЈ2ЃЉЁАбѕЛЏЁБЪБЃЌ1molAsO33-зЊЛЏЮЊAsO43-жСЩйашвЊO2______ molЁЃ
ЃЈ3ЃЉЁАГСЩщЁБЪЧНЋЩщдЊЫизЊЛЏЮЊCa5(AsO4)3OHГСЕэЃЌЗЂЩњЕФжївЊЗДгІгаЃК
aЃЎCa(OH)2ЃЈsЃЉ![]() Ca2+ЃЈaqЃЉ+2OH-ЃЈaqЃЉ ЁїHЃМ0
Ca2+ЃЈaqЃЉ+2OH-ЃЈaqЃЉ ЁїHЃМ0
bЃЎ5Ca2++OH-+3AsO43-![]() Ca5(AsO4)3OH ЁїHЃО0
Ca5(AsO4)3OH ЁїHЃО0
баОПБэУїЃКЁАГСЩщЁБЕФзюМбЮТЖШЪЧ85ЁцЁЃ гУЛЏбЇЦНКтдРэНтЪЭЮТЖШИпгк85ЁцКѓ,ЫцЮТЖШЩ§ИпГСЕэТЪЯТНЕЕФдвђЪЧ_____________________ЁЃ
ЃЈ4ЃЉЁАЛЙдЁБЙ§ГЬжаH3AsO4зЊЛЏЮЊH3AsO3ЃЌЗДгІЕФЛЏбЇЗНГЬЪНЪЧ_______________________ЁЃ
ЃЈ5ЃЉЁАЛЙдЁБКѓМгШШШмвКЃЌH3AsO3ЗжНтЮЊAs2O3ЃЌЭЌЪБНсОЇЕУЕНДжAs2O3ЁЃAs2O3дкВЛЭЌЮТЖШКЭВЛЭЌХЈЖШСђЫсжаЕФШмНтЖШЃЈSЃЉЧњЯпШчЭМ2ЫљЪОЁЃЮЊСЫЬсИпДжAs2O3ЕФГСЕэТЪЃЌЁАНсОЇЁБЙ§ГЬНјааЕФВйзїЪЧ_______ЁЃ
ЃЈ6ЃЉЯТСаЫЕЗЈжаЃЌе§ШЗЕФЪЧ ______ ЃЈЬюзжФИЃЉЁЃ
aЃЎДжAs2O3жаКЌгаCaSO4
bЃЎЙЄвЕЩњВњжаЃЌТЫвК2ПЩбЛЗЪЙгУЃЌЬсИпЩщЕФЛиЪеТЪ
cЃЎЭЈЙ§ЯШЁАГСЩщЁБКѓЁАЫсЛЏЁБЕФЫГађЃЌПЩвдДяЕНИЛМЏЩщдЊЫиЕФФПЕФ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЪЧвЛИіЛЏбЇЙ§ГЬЕФЪОвтЭМЁЃ
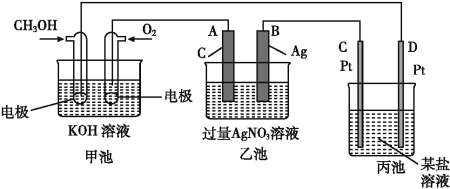
(1)C(Pt)ЕчМЋЕФУћГЦЪЧ____ЁЃ
(2)аДГіЭЈШыO2ЕФЕчМЋЩЯЕФЕчМЋЗДгІЪН:_______________ЁЃ
(3)аДГіЭЈШыCH3OHЕФЕчМЋЩЯЕФЕчМЋЗДгІЪН:_________ЁЃ
(4)ШєБћГиЪЧЕчНтБЅКЭЪГбЮЫЎШмвК,дк____(ЬюЁАбєМЋЁБЛђЁАвѕМЋЁБ)ИННќЕЮШыЗгЬЊШмвКБфКьЁЃ
(5)ввГижаЗДгІЕФЛЏбЇЗНГЬЪНЮЊ____ЁЃ
(6)ЕБввГижаB(Ag)МЋЕФжЪСПдіМг5.40 gЪБ,МзГижаРэТлЩЯЯћКФO2____mL(БъзМзДПіЯТ);ШєБћГижаБЅКЭЪГбЮЫЎШмвКЕФЬхЛ§ЮЊ500 mL,ЕчНтКѓ,ШмвКЕФpH=_____ЁЃ(25 Ёц,МйЩшЕчНтЧАКѓШмвКЕФЬхЛ§ЮоБфЛЏ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдквЛЖЈЬхЛ§ЕФУмБеШнЦїжаЃЌНјааШчЯТЛЏбЇЗДгІЃКCO2(g)+H2(g)CO(g)+H2O(g)ЃЌЦфЛЏбЇЦНКтГЃЪ§KКЭЮТЖШtЕФЙиЯЕШчБэЃК
t/Ёц | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
ЛиД№ЯТСаЮЪЬтЃК
(1)ИУЗДгІЕФЛЏбЇЦНКтГЃЪ§БэДяЪНЮЊK=___ЁЃвбжЊЃКK1000ЁцЃОK800ЁцЃЌдђИУЗДгІЪЧ__ЗДгІЁЃ(ЬюЁАЮќШШЁБЛђЁАЗХШШЁБ)ЃЛ
(2)вбжЊдк800ЁцЪБЃЌИУЗДгІЕФЦНКтГЃЪ§K1=0.9ЃЌдђИУЮТЖШЯТЗДгІCO(g)ЃЋH2O(g)![]() CO2(g)ЃЋH2(g)ЕФЦНКтГЃЪ§K2=___ЁЃ
CO2(g)ЃЋH2(g)ЕФЦНКтГЃЪ§K2=___ЁЃ
(3)ФмХаЖЯИУЗДгІЪЧЗёДяЕНЛЏбЇЦНКтзДЬЌЕФвРОнЪЧ__ЁЃ
AЃЎШнЦїжабЙЧПВЛБф
BЃЎЛьКЯЦјЬхжаc(CO)ВЛБф
CЃЎvе§(H2)=vФц(H2O)
DЃЎc(CO2)=c(CO)
(4)ФГЮТЖШЯТЃЌЦНКтХЈЖШЗћКЯЯТЪНЃКc(CO2)c(H2)=c(CO)c(H2O)ЃЌЪдХаЖЯДЫЪБЕФЮТЖШЮЊ__ЁцЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПСНЬзШчЭМЫљЪОзАжУЃЌЗжБ№ЮЊзАжУЂйКЭзАжУЂкЃЌИїЪЂга2gаПСЃ(ПХСЃДѓаЁЯрЭЌ)ЁЃ
ЪЕбщЂйЃКдкзАжУЂйжаМгШы40mL1mol/LЕФСђЫс
ЪЕбщЂкЃКдкзАжУЂкжаМгШы40mL4mol/LЕФСђЫсЁЃ
БШНЯЖўепЪеМЏ10mLH2ЪБЫљгУЕФЪБМфЁЃ
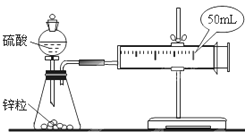
(1)ЕБЪеМЏЕН10mLH2ЪБЃЌФФИіЪЕбщЫљКФЪБМфНЯГЄЃП__(ЬюаДЪЕбщађКХ)ЮЊЪВУДЃП__ЁЃ
(2)ЛюШћЭтвЦЕФЧщПіЪЧ__ЁЃ
AЃЎОљдШЭтвЦ BЃЎЯШПьКѓТ§ CЃЎЯШТ§КѓПь DЃЎЯШТ§КѓПьЃЌШЛКѓгжж№НЅМѕТ§
ФубЁдёЕФРэгЩЪЧ__ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПТСМАЦфЛЏКЯЮядкЩњВњЩњЛюжаОпгаживЊЕФзїгУЁЃ
(1)ТСЪєгкЛюЦУН№ЪєШДФмдкПеЦјжаЮШЖЈДцдкЃЌдвђЪЧЃЈгУЛЏбЇгУгяМАЯрЙиЮФзжЫЕУїЃЉ___________
(2)ТСЕчГиадФмгХдНЃЌдкЯжДњЩњВњЁЂЩњЛюжагаЙуЗКЕФгІгУЁЃТС-ПеЦјЕчГивдЦфЛЗБЃЁЂАВШЋЖјЪмЕНдНРДдНЖрЕФЙизЂЃЌЦфдРэШчЯТЭМЫљЪОЁЃ
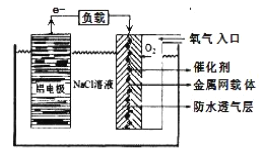
ИУЕчГиЕФе§МЋЗДгІЗНГЬЪНЮЊ _____ЃЛЕчГижаNaClШмвКЕФзїгУЪЧ ______ЃЛвдИУЕчГиЮЊЕчдДЃЌгУЖшадЕчМЋЕчНтNa2SO4ШмвКЃЌЕБAlЕчМЋжЪСПМѕЩй1.8gЪБЃЌЕчНтГивѕМЋЩњГЩЕФЦјЬхдкБъзМзДПіЯТЕФЬхЛ§ЮЊ_______LЁЃ
(3)AlCl3гыNaN3дкИпЮТЯТЗДгІПЩжЦЕУИпЮТНсЙЙЬеДЩЕЊЛЏТС(AlN)ЃЌЧвЩњГЩN2ЁЃNaN3ОЇЬхжавѕЁЂбєРызгИіЪ§БШЮЊ______ЃЌаДГіЗДгІЛЏбЇЗНГЬЪНЮЊ___________
(4)ЭЌжїзхЕФдЊЫигІгУЙуЗКЁЃ2019Фъ1дТ3ШеЩЯЮчЃЌцЯЖ№ЫФКХЬНВтЦїєцШЛТфдТЃЌЪзДЮЪЕЯжШЫРрЗЩааЦїдкдТЧђБГУцЕФШэзХТНЁЃЫљДюдиЕФЁАгёЭУЖўКХЁБдТЧђГЕЃЌЭЈЙ§ЩщЛЏяиЃЈGaAsЃЉЬЋбєФмЕчГиЬсЙЉФмСПНјааЙЄзїЁЃЛиД№ЯТСаЮЪЬтЃК
ЂйЛљЬЌGaдзгМлЕчзгХХВМЪН____ЃЌКЫЭтЕчзгеМОнзюИпФмМЖЕФЕчзгдЦаЮзДЮЊ____ЃЛЛљЬЌAsдзгзюИпФмВуЩЯга____ИіЕчзгЁЃ
ЂкяиЪЇШЅЕчзгЕФж№МЖЕчРыФмЃЈЕЅЮЛЃКkJ/molЃЉЕФЪ§жЕвРДЮЮЊ577ЁЂ1985ЁЂ2962ЁЂ6192ЃЌ-1гЩДЫПЩЭЦжЊяиЕФжївЊЛЏКЯМлЮЊ_____КЭ+3ЃЌЩщЕФЕквЛЕчРыФмБШяи_____ЬюЁАДѓЁБЛђЁАаЁЁБЃЉЁЃ
ЂлЕкЫФжмЦкдЊЫижаЃЌгыЛљЬЌAsдзгКЫЭтЮДГЩЖдЕчзгЪ§ФПЯрЭЌЕФдЊЫиЗћКХЮЊ____ЁЃ
ЂмЩщЛЏяиПЩгЩЃЈCH3ЃЉ3GaКЭAsH3дк700ЁцжЦЕУЃЌЃЈCH3ЃЉ3GaжаCдзгЕФдгЛЏЗНЪНЮЊ ______ЃЌAsH3ЗжзгЕФПеМфЙЙаЭЮЊ______ЁЃ
ЂнЯрЭЌбЙЧПЯТЃЌAsH3ЕФЗаЕу_______NH3ЃЈЬюЁАДѓгкЁБЛђЁАаЁгкЁБЃЉЃЌдвђЮЊ________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈ15ЗжЃЉКЌЬМЮяжЪЕФМлжЕаЭзЊЛЏЃЌгаРћгкЁАМѕЬМЁБКЭПЩГжајадЗЂеЙЃЌгазХживЊЕФбаОПМлжЕЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉвбжЊCOЗжзгжаЛЏбЇМќЮЊCЁдOЁЃЯрЙиЕФЛЏбЇМќМќФмЪ§ОнШчЯТЃК
ЛЏбЇМќ | HЁЊO | CЁдO | C=O | HЁЊH |
E/(kJЁЄmol1) | 463 | 1075 | 803 | 436 |
CO(g)ЃЋH2O(g)![]() CO2(g)ЃЋH2(g) ІЄH=___________kJЁЄmol1ЁЃЯТСагаРћгкЬсИпCOЦНКтзЊЛЏТЪЕФДыЪЉга_______________ЃЈЬюБъКХЃЉЁЃ
CO2(g)ЃЋH2(g) ІЄH=___________kJЁЄmol1ЁЃЯТСагаРћгкЬсИпCOЦНКтзЊЛЏТЪЕФДыЪЉга_______________ЃЈЬюБъКХЃЉЁЃ
aЃЎдіДѓбЙЧП bЃЎНЕЕЭЮТЖШ
cЃЎЬсИпдСЯЦјжаH2OЕФБШР§ dЃЎЪЙгУИпаЇДпЛЏМС
ЃЈ2ЃЉгУЖшадЕчМЋЕчНтKHCO3ШмвКЃЌПЩНЋПеЦјжаЕФCO2зЊЛЏЮЊМзЫсИљ(HCOO)ЃЌШЛКѓНјвЛВНПЩвджЦЕУживЊгаЛњЛЏЙЄдСЯМзЫсЁЃCO2ЗЂЩњЗДгІЕФЕчМЋЗДгІЪНЮЊ________________ЃЌШєЕчНтЙ§ГЬжазЊвЦ1 molЕчзгЃЌбєМЋЩњГЩЦјЬхЕФЬхЛ§ЃЈБъзМзДПіЃЉЮЊ_________LЁЃ
ЃЈ3ЃЉввБНДпЛЏЭбЧтжЦШЁБНввЯЉЕФЗДгІЮЊЃК![]() (g)ЃЋCO2(g)
(g)ЃЋCO2(g)![]()
![]() (g)ЃЋCO(g)ЃЋH2O(g)ЃЌЦфЗДгІРњГЬШчЯТЃК
(g)ЃЋCO(g)ЃЋH2O(g)ЃЌЦфЗДгІРњГЬШчЯТЃК
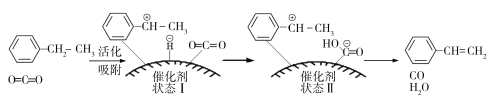
ЂйгЩдСЯЕНзДЬЌЂё____________ФмСПЃЈЬюЁАЗХГіЁБЛђЁАЮќЪеЁБЃЉЁЃ
ЂквЛЖЈЮТЖШЯТЃЌЯђКуШнУмБеШнЦїжаГфШы2 molввБНКЭ2 mol CO2ЃЌЦ№ЪМбЙЧПЮЊp0ЃЌЦНКтЪБШнЦїФкЦјЬхзмЮяжЪЕФСПЮЊ5 molЃЌввБНЕФзЊЛЏТЪЮЊ_______ЃЌгУЦНКтЗжбЙДњЬцЦНКтХЈЖШБэЪОЕФЛЏбЇЦНКтГЃЪ§Kp=_______ЁЃ[ЦјЬхЗжбЙ(pЗж)=ЦјЬхзмбЙ(pзм)ЁСЦјЬхЬхЛ§ЗжЪ§]
ЂлввБНЦНКтзЊЛЏТЪгыp(CO2)ЕФЙиЯЕШчЯТЭМЫљЪОЃЌЧыНтЪЭввБНЦНКтзЊЛЏТЪЫцзХp(CO2)БфЛЏЖјБфЛЏЕФдвђ________________________________ЁЃ

ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com