
ЁОЬтФПЁПСђЫсЪЧМЋЦфживЊЕФЛЏЙЄдСЯЃЌдкЙЄвЕЁЂХЉвЕЁЂвНвЉЁЂОќЪТЕШСьгђгІгУЙуЗКЁЃЙЄвЕЩЯЭЈГЃгУНгДЅЗЈжЦСђЫсЃЌжївЊдСЯЪЧСђЬњПѓКЭПеЦјЁЃНгДЅЗЈжЦСђЫсЕФЩњВњЙ§ГЬДѓжТПЩЗжЮЊШ§ИіНзЖЮЃКЖўбѕЛЏСђЕФжЦШЁКЭОЛЛЏЃЛЖўбѕЛЏСђзЊЛЏЮЊШ§бѕЛЏСђЃЛШ§бѕЛЏСђЕФЮќЪеКЭСђЫсЕФЩњГЩЁЃЮЊСЫЗРжЙЛЗОГЮлШОВЂЖдЮВЦјНјаазлКЯРћгУЃЌСђЫсГЇГЃгУАБЫЎЮќЪеЮВЦјЕФSO2ЁЂSO3ЕШЦјЬхЃЌдйЯђЮќЪевКжаМгШыХЈСђЫсЃЌвджЦШЁИпХЈЖШЕФSO2МАЃЈNH4ЃЉ2SO4КЭNH4HSO4ЙЬЬхЁЃЮЊСЫВтЖЈЩЯЪіЃЈNH4ЃЉ2SO4КЭNH4HSO4ЙЬЬхЛьКЯЮяЕФзщГЩЃЌЯжГЦШЁИУбљЦЗЫФЗнЃЌЗжБ№МгШыЯрЭЌХЈЖШЕФNaOHШмвК50.00mLЃЌМгШШжС120ЁцзѓгвЃЌЪЙАБЦјШЋВПвнГі[ЃЈNH4ЃЉ2SO4КЭNH4HSO4ЕФЗжНтЮТЖШОљИпгк200Ёц]ЃЌВтЕУгаЙиЪЕбщЪ§ОнШчЯТЃЈБъзМзДПіЃЉЃК
ЪЕбщ | бљЦЗЕФжЪСП/g | NaOHШмвКЕФЬхЛ§/mL | АБЦјЕФЬхЛ§/LЃЈБъзМзДПіЃЉ |
1 | 7.24 | 50.00 | 1.792 |
2 | 14.48 | 50.00 | 3.584 |
3 | 21.72 | 50.00 | 4.032 |
4 | 36.20 | 50.00 | 2.240 |
ЃЈ1ЃЉгЩ1зщЪ§ОнжБНгЭЦВтЃК1.81gбљЦЗНјааЭЌбљЪЕбщЪБЃЌЩњГЩАБЦјЕФЬхЛ§ЃЈБъзМзДПіЃЉЮЊ___LЁЃ
ЃЈ2ЃЉЪдМЦЫуИУЛьКЯЮяжаЃЈNH4ЃЉ2SO4КЭ NH4HSO4ЕФЮяжЪЕФСПжЎБШЮЊ___ЁЃ
ЃЈ3ЃЉЧѓЫљгУNaOHШмвКЕФЮяжЪЕФСПХЈЖШ___mol/LЁЃ
ЁОД№АИЁП0.448 1ЁУ2 6
ЁОНтЮіЁП
ЃЈNH4ЃЉ2SO4КЭ NH4HSO4ЕФЛьКЯвКжаМгШыЧтбѕЛЏФЦЃЌвРДЮЗЂЩњЕФЗДгІЪЧ2NH4HSO4+2NaOH=ЃЈNH4ЃЉ2SO4+Na2SO4+2H2OЁЂЃЈNH4ЃЉ2SO4+2NaOH= Na2SO4+2H2O+2NH3ЁЃ
ЃЈ1ЃЉИљОнЪЕбщ1ЁЂ2ПЩжЊЃЌдіМгбљЦЗЕФжЪСПЃЌЗХГіЕФАБЦјдіЖрЃЌЫЕУїЪЕбщ1жаЧтбѕЛЏФЦЙ§СПЃЌ7.24gбљЦЗжаЕФяЇИљРызгШЋВПЩњГЩАБЦјЗХГіЃЌШєМгШы1.81gбљЦЗЃЌяЇИљРызгвВФмШЋВПЩњГЩАБЦјЗХГіЃЌЩшЗХГіАБЦјЕФЬхЛ§ЪЧVLЃЌдђ![]() ЃЌV=0.448LЁЃ
ЃЌV=0.448LЁЃ
ЃЈ2ЃЉЪЕбщ1жаЃЌ7.24gбљЦЗжаЕФяЇИљРызгШЋВПЩњГЩАБЦјЗХГіЃЌЩшбљЦЗжаЕФЃЈNH4ЃЉ2SO4КЭ NH4HSO4ЮяжЪЕФСПЗжБ№ЪЧxmolЁЂymolЃЌИљОнЕЊдЊЫиЪиКуЃЌ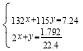 ЃЌНтЕУx=0.02molЁЂy=0.04molЃЌ
ЃЌНтЕУx=0.02molЁЂy=0.04molЃЌ![]() ЃЛ
ЃЛ
ЃЈ2ЃЉгЩЪЕбщ3ЁЂ4ПЩвдПДГіЃЌЪЕбщ3дйдіДѓбљЦЗЕФжЪСПЃЌЩњГЩАБЦјЕФЬхЛ§МѕаЁЃЌЫЕУїЧтбѕЛЏФЦЮяжЪЕФСПВЛзуЃЌЩш36.2gбљЦЗжаNH4HSO4ЮяжЪЕФСПЮЊamolЃЌдђ![]() ЃЌa=0.2molЃЌЯШЗЂЩњЗДгІH++OH-=H2OЃЌдђИУЗДгІЯћКФЧтбѕЛЏФЦЕФЮяжЪЕФСПЮЊ0.2molЃЌдйЗЂЩњNH4++OH-
ЃЌa=0.2molЃЌЯШЗЂЩњЗДгІH++OH-=H2OЃЌдђИУЗДгІЯћКФЧтбѕЛЏФЦЕФЮяжЪЕФСПЮЊ0.2molЃЌдйЗЂЩњNH4++OH-![]() ЃЌИљОнАБЦјЕФЬхЛ§ЮЊ2.24LЃЌИУЗДгІЯћКФЧтбѕЛЏФЦЕФЮяжЪЕФСПЮЊ0.1molЃЌЫљвд50mLЧтбѕЛЏФЦШмвККЌгаЧтбѕЛЏФЦЕФЮяжЪЕФСПЪЧ0.3molЃЌЧтбѕЛЏФЦШмвКЕФХЈЖШЪЧ
ЃЌИљОнАБЦјЕФЬхЛ§ЮЊ2.24LЃЌИУЗДгІЯћКФЧтбѕЛЏФЦЕФЮяжЪЕФСПЮЊ0.1molЃЌЫљвд50mLЧтбѕЛЏФЦШмвККЌгаЧтбѕЛЏФЦЕФЮяжЪЕФСПЪЧ0.3molЃЌЧтбѕЛЏФЦШмвКЕФХЈЖШЪЧ![]() 6mol/LЁЃ
6mol/LЁЃ


 ПкЫуЬтПЈМггІгУЬтМЏбЕЯЕСаД№АИ
ПкЫуЬтПЈМггІгУЬтМЏбЕЯЕСаД№АИ злКЯздВтЯЕСаД№АИ
злКЯздВтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПH2O2ЪЧЪЕбщЪвГЃМћЕФЧПбѕЛЏМСЃЌдквНСЦЩЯПЩгУзїЯћЖОМСЕШЁЃ
ЃЈ1ЃЉвЛжже§дкПЊЗЂЕФРћгУO2КЭH2OзїдСЯЭЈЙ§ЛЏКЯжЦШЁH2O2ЕФЗНЗЈЃЌЦфдРэШчЭМЫљЪОЁЃИУЗНЗЈжЦШЁH2O2ЕФзмЛЏбЇЗДгІЗНГЬЪНЮЊ____ЁЃ
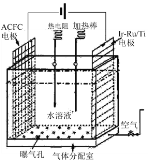
ЃЈ2ЃЉЮЊЬНОПЭтНчЬѕМўЖдH2O2ЗжНтЛЏбЇЗДгІЫйТЪЕФгАЯьЃЌЯрЙиЪЕбщЩшМЦШчЯТБэЫљЪОЃК
ЪдЙм БрКХ | ЪЕбщФПЕФ | H2O2ШмвК | ЮТЖШ | ЫЎЕФ ЬхЛ§/mL | FeCl3ШмвКЬхЛ§/mL | |
жЪСП ЗжЪ§ | ЬхЛ§/mL | |||||
Ђё | ЮЊБрКХЂђЪЕбщВЮее | 12% | 5ЃЎ0 | ГЃЮТ | 0 | 0 |
Ђђ | ЮТЖШЖдЗДгІЫйТЪЕФгАЯь | ЃЈ ЃЉ | 5ЃЎ0 | 60Ёц | 0 | 0 |
Ђѓ | ЮЊБрКХЂєЪЕбщВЮее | 4ЃЎ0% | 5ЃЎ0 | ГЃЮТ | ЃЈ ЃЉ | 0 |
Ђє | ЃЈ ЃЉ | 4ЃЎ0% | 5ЃЎ0 | ГЃЮТ | 0 | 1ЃЎ0 |
ЬюаДБэжаШБЩйЕФФкШнЃКЂђ_______ЃЛЂѓ__________ЃЛЂє_________ЁЃ
ЃЈ3ЃЉгЩВЌЃЈPtЮЛгкзѓБпЃЉКЭН№ЃЈAuЮЛгкгвБпЃЉзщГЩЕФФЩУзАєЗХШыH2O2ШмвКжаЃЈШчЭМЃЉЃЌФЩУзАєНЋЗЂЩњЖЈЯђвЦЖЏЁЃ
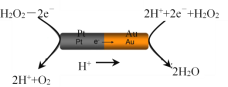
дђЃКAuвЛВрЮЊЕчГиЕФ____МЋЃЈбЁЬюЃКЁАе§ЁБЛђЁАИКЁБЃЉЃЛФЩУзАєЯђ____ЃЈбЁЬюЃКЁАзѓЁБЛђЁАгвЁБЃЉвЦЖЏЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЁАвдЗЯжЮЗЯЁБЪЧЛљгкЁАТЬЩЋЛЏбЇЁБЙлФюжЮРэЮлШОЕФЫМТЗЁЃгУЙЄвЕЗЯМюдќЃЈжївЊГЩЗжЮЊNa2CO3ЃЉЮќЪебЬЦјжаЕФSO2ЃЌЕУЕНбЧСђЫсФЦЃЈNa2SO3ЃЉДжЦЗЁЃЦфСїГЬШчЯТЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ
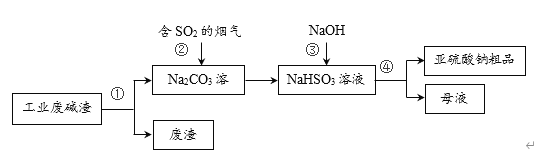
A.ВйзїЂйЁЂЂмОљЮЊЙ§ТЫ
B.ВНжшЂкжаЗЂЩњСЫжУЛЛЗДгІ
C.ВНжшЂлЗЂЩњЕФЗДгІЮЊЃКNaHSO3ЃЋNaOH = Na2SO3ЃЋH2O
D.бЧСђЫсФЦДжЦЗжаВЛПЩФмКЌгаNa2SO4
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГбЇЯАаЁзщгУЯТЭМзАжУбаОПSO2ЕФаджЪЁЃ
| ађКХ | X | ЪЕбщЯжЯѓ |
Ђё | зЯЩЋЪЏШяШмвК | ЯжЯѓa | |
Ђђ | ЦЗКьШмвК | ШмвКгЩКьЩЋБфЮЊЮоЩЋЃЌМгШШКѓгжЛжИДдРДЕФбеЩЋ | |
Ђѓ | ЫсадKMnO4ШмвК | ШмвКгЩзЯЩЋБфЮЊЮоЩЋ |
ЧыЛиД№ЃК
ЃЈ1ЃЉЪЕбщЂёжаЃЌЯжЯѓaЪЧ______ЁЃ
ЃЈ2ЃЉИљОнЪЕбщЂђЃЌЭЦЖЯSO2ЕФЛЏбЇаджЪЪЧ______ЁЃ
ЃЈ3ЃЉИљОнЪЕбщЂѓЃЌЭЦЖЯЮоЩЋШмвКжаЫљКЌЕФРызгЪЧK+ЁЂMn2+ЁЂH+КЭ______ЁЃ
ЃЈ4ЃЉНсКЯРызгЗНГЬЪНЫЕУїЪЕбщжаNaOHШмвКЕФзїгУЪЧ______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП(1)гавЛЦПЮоЩЋГЮЧхШмвКЃЌЦфжаПЩФмКЌH+ЁЂNa+ЁЂMg2+ЁЂBa2+ЁЂClЁЂSO42ЁЂCO32РызгЁЃЯжНјаавдЯТЪЕбщЃК
AЁЂгУpHЪджНМьбщШмвКЃЌЗЂЯжШмвКГЪЧПЫсадЃЛ
BЁЂШЁВПЗжШмвКж№ЕЮМгШыNaOHШмвКЃЌЪЙШмвКгЩЫсадБфЮЊМюадЃЌЮоГСЕэВњЩњЃЛ
CЁЂШЁЩйСПBжаЕФМюадШмвКЃЌЕЮМгNa2CO3ШмвКЃЌгаАзЩЋГСЕэВњЩњЁЃ
ЂйИљОнЩЯЪіЪТЪЕШЗЖЈЃКИУШмвКжаПЯЖЈДцдкЕФРызгга_________________________ЃЛ
ПЯЖЈВЛДцдкЕФРызгга___________________________ЁЃ
ЂкаДГіCжаЗЂЩњЗДгІЕФРызгЗНГЬЪН________________________________ЁЃ
(2)ЂйЛЙдЬњЗлгыИпЮТЫЎеєЦјЗДгІЕФЛЏбЇЗНГЬЪНЃК_____________________________ЃЛ
ЂкГ§ШЅMgЗлжаЕФAlЗлЕФЪдМСЪЧ__________________ЃЌЗДгІЕФРызгЗНГЬЪНЮЊЃК___________________________________ЃЛ
(3)ИпЬњЫсФЦЃЈNa2FeO4ЃЉОпгаЧПбѕЛЏадЃЌПЩЖдздРДЫЎНјааЯћЖОЁЂОЛЛЏЁЃИпЬњЫсФЦПЩгУЧтбѕЛЏЬњКЭДЮТШЫсФЦдкМюадНщжЪжаЗДгІЕУЕНЃЌЧыВЙГфВЂХфЦНЯТУцРызгЗНГЬЪНЁЃ
____Fe(OH)3 +____ClOЃ+____OHЃ =__FeO42ЃЃЋ___ClЃ+_____ _______
(4)дкЗДгІ11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4жаЃЌбѕЛЏМСЪЧ___________ЃЛ
ЕБга2mol H3PO4ЩњГЩЃЌзЊвЦЕФЕчзгЕФЮяжЪЕФСПЮЊ__________________.
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈВЛе§ШЗЕФЪЧЃЈ ЃЉ
A.35ClКЭ37ClЛЅЮЊЭЌЮЛЫи
B.ввЫсКЭгЭЫсЃЈC17H33COOHЃЉЛЅЮЊЭЌЯЕЮя
C.КьСзКЭАзСзЛЅЮЊЭЌЫивьаЮЬх
D.ввШЉКЭЛЗбѕввЭщ(![]() )ЛЅЮЊЭЌЗжвьЙЙЬх
)ЛЅЮЊЭЌЗжвьЙЙЬх
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПСђЫсЪЧМЋЦфживЊЕФЛЏЙЄдСЯЃЌдкЙЄвЕЁЂХЉвЕЁЂвНвЉЁЂОќЪТЕШСьгђгІгУЙуЗКЁЃЙЄвЕЩЯЭЈГЃгУНгДЅЗЈжЦСђЫсЃЌжївЊдСЯЪЧСђЬњПѓКЭПеЦјЁЃНгДЅЗЈжЦСђЫсЕФЩњВњЙ§ГЬДѓжТПЩЗжЮЊШ§ИіНзЖЮЃКЖўбѕЛЏСђЕФжЦШЁКЭОЛЛЏЃЛЖўбѕЛЏСђзЊЛЏЮЊШ§бѕЛЏСђЃЛШ§бѕЛЏСђЕФЮќЪеКЭСђЫсЕФЩњГЩЁЃЮЊСЫЗРжЙЛЗОГЮлШОВЂЖдЮВЦјНјаазлКЯРћгУЃЌСђЫсГЇГЃгУАБЫЎЮќЪеЮВЦјЕФSO2ЁЂSO3ЕШЦјЬхЃЌдйЯђЮќЪевКжаМгШыХЈСђЫсЃЌвджЦШЁИпХЈЖШЕФSO2МАЃЈNH4ЃЉ2SO4КЭNH4HSO4ЙЬЬхЁЃЮЊСЫВтЖЈЩЯЪіЃЈNH4ЃЉ2SO4КЭNH4HSO4ЙЬЬхЛьКЯЮяЕФзщГЩЃЌЯжГЦШЁИУбљЦЗЫФЗнЃЌЗжБ№МгШыЯрЭЌХЈЖШЕФNaOHШмвК50.00mLЃЌМгШШжС120ЁцзѓгвЃЌЪЙАБЦјШЋВПвнГі[ЃЈNH4ЃЉ2SO4КЭNH4HSO4ЕФЗжНтЮТЖШОљИпгк200Ёц]ЃЌВтЕУгаЙиЪЕбщЪ§ОнШчЯТЃЈБъзМзДПіЃЉЃК
ЪЕбщ | бљЦЗЕФжЪСП/g | NaOHШмвКЕФЬхЛ§/mL | АБЦјЕФЬхЛ§/LЃЈБъзМзДПіЃЉ |
1 | 7.24 | 50.00 | 1.792 |
2 | 14.48 | 50.00 | 3.584 |
3 | 21.72 | 50.00 | 4.032 |
4 | 36.20 | 50.00 | 2.240 |
ЃЈ1ЃЉгЩ1зщЪ§ОнжБНгЭЦВтЃК1.81gбљЦЗНјааЭЌбљЪЕбщЪБЃЌЩњГЩАБЦјЕФЬхЛ§ЃЈБъзМзДПіЃЉЮЊ___LЁЃ
ЃЈ2ЃЉЪдМЦЫуИУЛьКЯЮяжаЃЈNH4ЃЉ2SO4КЭ NH4HSO4ЕФЮяжЪЕФСПжЎБШЮЊ___ЁЃ
ЃЈ3ЃЉЧѓЫљгУNaOHШмвКЕФЮяжЪЕФСПХЈЖШ___mol/LЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЙЬЬхXПЩФмКЌгаNa2O2ЁЂFe2O3ЁЂAl2O3ЁЂSiO2ЁЂK2SO4ЁЂNa2SO3ЁЂNH4NO3ЁЂMgCl2жаЕФвЛжжЛђМИжжЮяжЪЃЌНјааШчЯТЪЕбщвдШЗЖЈЦфзщГЩЃК
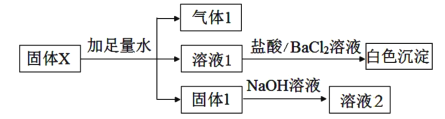
ЯТСаЫЕЗЈВЛе§ШЗЕФЪЧЃЈ ЃЉ
A.ШмвК1жаВЛПЩФмКЌгаCl-
B.ЦјЬх1ПЩФмЪЧЖўжжЦјЬхЕФЛьКЯЮя
C.ЙЬЬх1ПЩФмЪЧЖўжжЙЬЬхЕФЛьКЯЮя
D.ЙЬЬхXжаЃЌK2SO4КЭNa2SO3СНжжЮяжЪжСЩйКЌвЛжж
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжгаН№ЪєЕЅжЪAЁЂBЁЂCКЭЦјЬхМзЁЂввЁЂБћвдМАЮяжЪDЁЂEЁЂFЁЂGЁЂHЃЌЫќУЧжЎМфЕФЯрЛЅзЊЛЏЙиЯЕШчЭМЫљЪОЃЈЭМжагааЉЗДгІЕФЩњГЩЮяКЭЗДгІЕФЬѕМўУЛгаБъГіЃЉЁЃЧыИљОнвдЩЯаХЯЂЭъГЩЯТСаИїЬтЃК
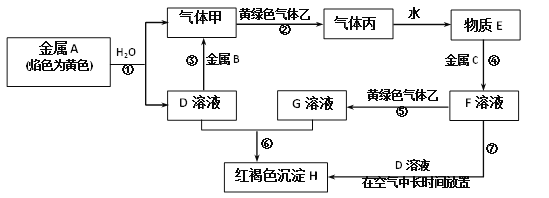
(1)аДГіЯТСаЮяжЪЕФЛЏбЇЪНЃКB_______ЁЂБћ_______ЁЃ
(2)аДГіЛЦТЬЩЋЦјЬхввЕФвЛжжгУЭО_______ЃЌЗДгІЙ§ГЬЂпПЩФмЙлВьЕНЕФЪЕбщЯжЯѓЪЧ________ЁЃ
(3)аДГіЗДгІЂпжаЩцМАЕФЛЏбЇЗДгІЗНГЬЪНЃК_______ЁЂ________ЁЃ
(4)аДГіЗДгІЂнЕФРызгЗНГЬЪН____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com