
(12��)������������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��ŨH2SO4������500 mL 0.2 mol��L��1��ϡH2SO4���ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� ��ҩ�� ����Ͳ ������ƿ ��������ƽ��
��ش��������⣺
��1�����������У�������ϡH2SO4ʱ�ò������� (�����)������ʱ��Ƿȱ�������� ��
��2�������㣬��ŨH2SO4�����Ϊ mL����ȷ��0.1����
��3�����ƹ��������²�����
A����Һ
B����ȡ
C��ϴ��
D������
E���ܽ�
F��ҡ��
����ȷ�IJ���˳��Ӧ�� (�����)��
��4�������ƹ����У�����������ȷ�����в����У����������ƫ�ߵ��� (�����)��
��ϴ����ȡŨH2SO4�����Ͳ������ϴ��Һת�Ƶ�����ƿ��
��ϡ�ͺ��H2SO4��Һδ����ȴ�����¾�ת�Ƶ�����ƿ��
�۶���ҡ�Ⱥ���Һ����ڱ��ߣ����ý�ͷ�ιܼ�����ˮ������
��ת��ǰ������ƿ�к�����������ˮ
�ݶ���ʱ�����ӱ���
��5������������ƿ����ȡ25.00mL��ϡ������Һ��100mL������ƿ����ˮϡ�����̶��ߡ�����������Һ��c��H+��= ��
��6��ij�о�С�������������Ͷ�������ͨ��ˮ��Һ�����Ʊ�100mL��0.4molH+����Һ����Ӧԭ����Cl2+SO2+2H2O= H2SO4+2HCl���������Ʊ��������������ģ��������״���µ����� L��
��1����2�֣�ÿ��1�֣��ڢ� �� ��ͷ�ι�
��2����2�֣�5.4mL ��3����2�֣� B E A C A D F ��4����2�֣� �٢ڢ�
��5����2�֣�0.1mol/L ��6����2�֣�2.24
��������
�����������1�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ�����������ձ�����ͷ�ιܡ���Ͳ������ƿ������Ҫ����������ƿ��������ƽ��ҩ�ף����Դ�Ϊ�ڢ� �ߣ�����ʱ��ȱ�ٽ�ͷ�ιܡ���2������Ҫ98%H2SO4�����ΪVmL��������Һϡ��ǰ����������������VmL��1.84g/cm3��98%��500mL��0.2mol?L-1��98g/mol�����V=5.4ml����3�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ�����Բ���˳��ΪB E A C A D F����4����ϴ����ȡŨH2SO4�����Ͳ������Ҫ��ϴ��Һת�Ƶ�����ƿ�У�����ʵ����ƫ�ߣ���ϡ�ͺ��H2SO4��ҺӦ��ȴ�����²���ת�Ƶ�����ƿ��������ȴ������ƿ����Һ������٣�Ũ��ƫ�ߣ��۶���ҡ�Ⱥ���Һ����ڱ��ߣ����ý�ͷ�ιܼ�����ˮ�����ߣ������ᵼ����Һ�����ƫ���ƫ�͢�ת��ǰ������ƿ�к�����������ˮ����ʵ����Ӱ��ݶ���ʱ�����ӱ��ߣ���ʹ��Һ�����ƫС�����ƫ�ߡ���5��ȡ����Һϡ����100/25=4������Ũ��Ϊԭ����1/4����0.2��2/4=0.1molL����6���Ʊ�100mL��0.4molH+����Һ���ɷ���ʽ����֪��ÿ����1mol��������������4mol�������ӣ�����Ҫ0.1mol���������ڱ�״����2.24L������
���㣺������Һ�����ơ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�걱���и߶��ϵ�һ��������⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��������ƣ�Na2S2O3����ϡH2SO4��Һʱ�������·�Ӧ��
Na2S2O3+ H2SO4=Na2 SO4+SO2+S��+H2O ���з�Ӧ����������
A��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ5mL����Ӧ�¶�10��
B��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�10��
C��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�30��
D��0.2mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�30��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��Ϫ�и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
(10��)��ͼ�ǹ�ҵ��������淋����̡�
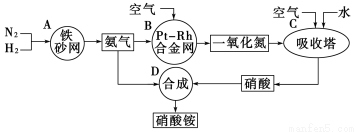
��1��������C��ͨ�������Ŀ����__________________ ��A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ����________(����ĸ)��
��2����֪��4NH3(g)��3O2(g)===2N2(g) ��6H2O(g) ��H����1266.8 kJ/mol
N2(g)��O2(g)===2NO(g) ��H����180.5 kJ/mol
д�������´��������Ȼ�ѧ����ʽ��____________________________��
��3����֪��N2(g) ��3H2(g)  2NH3(g) ��H����92 kJ/mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ��______________(����ĸ)��
2NH3(g) ��H����92 kJ/mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ��______________(����ĸ)��
A�������¶� B��ʹ�ô���
C������ѹǿ D��ѭ�����úͲ��ϲ��䵪��
��4����һ���¶Ⱥ�ѹǿ�£���H2��N2��3 : 1(�����)���ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������NH3�������������Ϊ17.6%�����ʱH2��ת���ʣ�(Ҫ�������ļ�����̣����������λ��Ч����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��Ϫ�и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ�������£�����A2(g)��3B2(g) 2AB3(g)��Ӧ��˵�����»�ѧ��Ӧ���ʵı�ʾ�У���ѧ��Ӧ����������
2AB3(g)��Ӧ��˵�����»�ѧ��Ӧ���ʵı�ʾ�У���ѧ��Ӧ����������
A��v(A2)��0.8 mol��L��1��s��1 B��v(A2)��30 mol��L��1��min��1
C��v(AB3)��1.0 mol��L��1��s��1 D��v(B2)��1.2 mol��L��1��s��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��Ϫ�и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ӦNH4HS(s) NH3(g)��H2S(g)��ij�¶��´ﵽƽ�⣬���и�������У�����ʹƽ�ⷢ���ƶ�����
NH3(g)��H2S(g)��ij�¶��´ﵽƽ�⣬���и�������У�����ʹƽ�ⷢ���ƶ�����
A���¶ȡ��ݻ�����ʱ��ͨ��SO2����
B������һ����NH4HS����
C���ݻ����䣬���백��
D������ѹǿ���䣬���뵪��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��Ϫ�и�һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ�У���ȷ����
A������ϡ���ᷴӦ��2Fe+6H+=2Fe3++3H2��
B��������Һ�м���Ba(OH)2��Һ��SO42- + Ba2��= BaSO4��
C����ϡ����ϴȥ���⣺Fe2O3+6H+=2Fe3++3H2O
D������Ƭ��������ͭ��Һ�У�Cu2++Al=Al3++Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��Ϫ�и�һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A����ij��Һ�м���Ba(NO3)2��Һ�������ɫ��������ԭ��Һ��һ������SO42��
B����ijϡ��Һ�м���Ba(NO3)2��Һ�������������ٵ��뼸�������ữ��AgNO3��Һ��������ɫ������˵��һ�����Ȼ������Һ
C����ʢ��H2��С�Թܹܿ����Ͽ����ƾ��ƻ������H2�Ĵ���
D����ȼ�ŵ�ľ���������ܿڣ�ľ��Ϩ��˵����CO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ��Ϫ�и�һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͭ��������ӻ�ͭ��ʼ����֪��ͭ������Ϊ+2�ۡ���ͭ��ı��չ�������Ҫ��Ӧ֮һ�Ļ�ѧ����ʽΪ��2CuFeS2+O2=Cu2S+2FeS+SO2������˵������ȷ����
A��O2ֻ��������
B��CuFeS2�������������ǻ�ԭ��
C��SO2���������������ǻ�ԭ����
D������1mol O2�μӷ�Ӧ����Ӧ����4mol����ת��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ���Ϻ�����������У��һ��ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
XԪ��ԭ�ӵĺ˵����Ϊn��X2�����Ӻ�Y3+���ӵĵ��Ӳ�ṹ��ͬ����Yԭ�ӵ�������Ϊ
A��n+1 B��n+2 C��n+3 D��n+5
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com