
����Ŀ���ڲⶨ����ͭ�����нᾧˮ������ʵ������У�
��1������ǰӦ���������__________�����飬�����Ƿ���__________�н��У�����ʧˮ��Ӧ����__________����ȴ��
��2���ж��Ƿ���ȫʧˮ�ķ�����______________________________________________��
��3��������ijѧ��һ��ʵ������ݣ�����ɼ��㣬��������ı����С�
�������� | �����뾧�������� | ���Ⱥ���������������� | ��þ����нᾧˮ���� |
11.7 g | 22.7 g | 17. 6 g | _________ |
��4�����ʵ���в�������ԭ�������__________����д��ĸ������ɡ�
A������ͭ�����к����ӷ�����־ B��ʵ��ǰ�����Ѳ��ֱ��
C������ʱ���岿�ֱ�� D������ʧˮ��©���ڿ�������ȴ
��5����֪�������м�������ͭ���壬���ȷֽ�������£�
CuSO4��5H2O![]() CuSO4��3H2O
CuSO4��3H2O ![]() CuSO4��H2O
CuSO4��H2O ![]() CuSO4
CuSO4
���˽�����ͼ���װ�ý�������ͭ������ˮʵ�飬�ش��������⣺
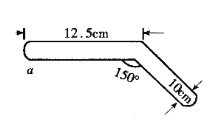
�ٱ�ʵ���������֤�Ļ�ѧ������_____________________________��
��a������Ƭ�̺�����______________________________________��
������Ϊ��װ������Ƿ��������ѧ���粻��������д�����ɣ�___________________________________________________________________________________________________��
���𰸡��в� ���� ������ ���������μ��ȵ����������0.1 g����˵����ȫʧˮ 7.7 ACD �����غ㶨�� ��ɫ�����Ϊ��ɫ�� ������������������˼��ȡ�
��������
����ͭ�����нᾧˮ������������![]() (�ᾧˮ������=����ͭ����ʹ���������������ˮ����ͭ�ʹ����������� )������Ϊ����ĥ�����в��н�����ͭ�������顣�ڳ�����ȷ��������Ĵ����������������ô�����ȷ��ȡһ�����������������ͭ���塣�ۼ��ȣ����Ⱦ��壬ʹ��ʧȥȫ���ᾧˮ(����ɫ��ȫ��Ϊ��ɫ)���ܳ������ڸ���������ȴ������������´���������ˮ����ͭ�����������ټ��ȡ��ٳ��������أ���ʢ����ˮ����ͭ�Ĵ������ټ��ȣ��ٷ������������ȴ���ٳ������������������������γ���������������0.1gΪֹ�����㣺����ʵ���õĽ��������ͭ�����нᾧˮ������������
(�ᾧˮ������=����ͭ����ʹ���������������ˮ����ͭ�ʹ����������� )������Ϊ����ĥ�����в��н�����ͭ�������顣�ڳ�����ȷ��������Ĵ����������������ô�����ȷ��ȡһ�����������������ͭ���塣�ۼ��ȣ����Ⱦ��壬ʹ��ʧȥȫ���ᾧˮ(����ɫ��ȫ��Ϊ��ɫ)���ܳ������ڸ���������ȴ������������´���������ˮ����ͭ�����������ټ��ȡ��ٳ��������أ���ʢ����ˮ����ͭ�Ĵ������ټ��ȣ��ٷ������������ȴ���ٳ������������������������γ���������������0.1gΪֹ�����㣺����ʵ���õĽ��������ͭ�����нᾧˮ������������
��1������ǰӦ����������в������飬�����Ƿ��������н��У�����ʧˮ��Ӧ���ڸ���������ȴ��
��2���ж��Ƿ���ȫʧˮ�ķ��������������μ��ȵ����������0.1 g����˵����ȫʧˮ��
��3���ᾧˮ������=����ͭ����ʹ���������������ˮ����ͭ�ʹ�����������=22.7g-17.6g=5.1g���ᾧˮ�ĸ���=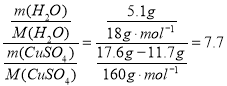 ��
��
��4��A������ͭ�����к����ӷ�����־�����¼��ȹ����й�����������࣬�ⶨ���ƫ��A��ȷ��
B��ʵ��ǰ�����Ѳ��ֱ��ʵ�鲻Ӱ�죬B����
C������ʱ���岿�ֱ�ڣ�˵��CuSO4�ѷ����ֽ�CuSO4![]() CuO+SO3����ʹ�ⶨ�Ľ��ƫ��C��ȷ��
CuO+SO3����ʹ�ⶨ�Ľ��ƫ��C��ȷ��
D������ʧˮ��©���ڿ�������ȴ���������տ����е�ˮ���ǹ�����ٵIJ�ֵ���ͣ��ⶨ���ƫС��D��ȷ��
��ΪACD��
��5�����װ�ý�������ͭ������ˮʵ�飬��ʵ�������ʲ���ɢʧ����ʵ���������֤�Ļ�ѧ�����������غ㶨�ɡ�
��a������Ƭ�̺ᾧˮ�ֽ⣬����Ϊ��ɫ�����Ϊ��ɫ�ۣ�
�۴�װ�ò�����������������˼��ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȩ���Ʊ����ᡢ����������Ȼ�����Ʒ��ԭ�ϡ����������գ�
(1)��ȩ�����еĹ�����Ϊ______��
(2)��ͭ˿�ڿ��������ձ�ں�Ѹ�������Ҵ��У��۲쵽ͭ˿����______������������β������ŵ��̼�����ζ��˵����______���ɡ�
(3)д��������ȩ�Ļ�ѧ��Ӧ����ʽ��______������Ӧ��ʾ��ȩ����______�ԡ�
(4)��֪����Ҳ�ܷ���������Ӧ����ij������Һ�п��ܻ�����ȩ�����ͨ��ʵ��֤���Ƿ�����ȩ��д����Ҫ��������________________
(5)��֪���л���ѧ�н����ڹ����ŵĵ�һ��̼ԭ�ӳ�Ϊ����C������C�ϵ�H�ͳ�Ϊ����H��ȩ������H�ϻ��ã����Ժ���һ��ȩ���ʻ����мӳɣ������ǻ�ȩ���磺
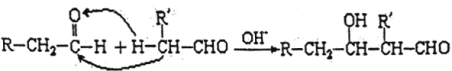
���һ������ϩΪԭ���Ʊ�������CH3CH2CH2CH2OH�ĺϳ�·��(���Լ���ѡ)��_________(�ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B����
B����![]() Ŀ�����)
Ŀ�����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����õ��ԭ����SO2��NOxת��Ϊ��NH4��2SO4��װ����ͼ��ʾ������˵��������ǣ� ��
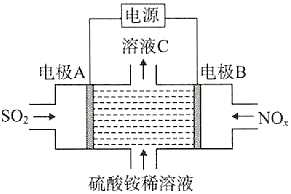
A.�����ĵ缫��Ӧʽ��NOx+��2x+3��e-+��2x+4��H+=NH4++xH2O
B.��ҺC�����Ա������ϡ��Һǿ
C.�缫A���Դ�ĸ�������������������Ӧ
D.ת��0.2mol����ʱ����0.1molSO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڵ��µĸֹܵ���������ͼ��ʾ�������е绯ѧ����������˵����ȷ���ǣ� ��
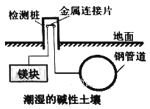
A.�ֹܵ����淢���˻�ԭ��Ӧ
B.�÷���������ת��Ϊ�˻�ѧ��
C.�÷�����Ϊ��ӵ�������������
D.þ���Ϸ����ĵ缫��Ӧ��O2+2H2O+4e��4OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС���ڳ����²ⶨһ��������ijͭ���������ͭ���������������������ʵ�鷽����
����I��ͭ�������![]() �ⶨ������������
�ⶨ������������
����II��ͭ�������![]() �ⶨʣ����������
�ⶨʣ����������
�����й��ж��в���ȷ���ǣ� ��
A.��ҺA��B������������
B.��ҺA��B��������NaOH��Һ
C.��ҺA��B��ֻ�������ᣬ��������NaOH��Һ
D.ʵ���ҷ���II������ʵʩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��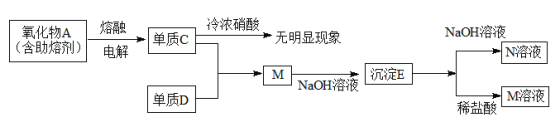
(1)M�Ļ�ѧʽ��AlCl3�������ӷ���ʽ��ʾ��ˮ��Һ����������______________��
(2)Ԫ��C�����ڱ��е�λ���ǵ������ڵڢ�A�壬������������Ӧ��ˮ����ĵ��뷽��ʽ��____________________��
(3)д��E��NaOH��Һ��Ӧ�����ӷ���ʽ________________________��
(4)����C����Ũ���������������ԭ����______________________��
(5)�������̬������Aʱ�����������ĵ缫��Ӧ��___________________��û�з����ۼ�֮ǰ������������A�۵�ߣ�����ʵ�ֹ�ҵ�����̣������ʽṹ�Ƕ�˵��A���и��۵��ԭ��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.20 mol/L����������ʵ���Ũ��Ϊc mol/L��NaOH��Һ����ͬ��������Ƴ�������Һ���±�������ʱ��ȡ������ NaOH ��Һ������Ϻ���Һ��Na+��Cl-�����ʵ���Ũ�����ݣ�������Һ����仯����
��Һ | ���ǰ��ȡ��Һ�����mL�� | ��Ϻ�����Ũ�ȣ�mol/L�� | ||
HCl | NaOH | Na+ | Cl- | |
�� | 30 | x | 1.5z | z |
�� | 10 | y | z | 2z |
����˵����ȷ�� ��
A.x=90B.y=30C.z=0.10D.c=0.10
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������仯����������������ռ�м�����Ҫ�ĵ�λ�����Ͻ������仯��������֪ʶ�ش��������⡣
��1����̼����0.03%2%֮��ij�Ͻ���Ŀǰʹ�������ĺϽ����ֺϽ���___��
A.���Ͻ� B.��ͭ C.þ�Ͻ� D.��
��2��FeCl3��Һ���ڸ�ʴͭ��ӡˢ��·�壬��Ӧ�����ӷ���ʽΪ___��
��3��ij��Һ����Mg2+��Fe2+��Al3+��Cu2+�����ӣ������м��������Na2O2���ˣ�������Ͷ�������������У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������__(����ĸ)���������ӵ�������___���ѧʽ����
A.Mg2+ B.Fe2+ C.Al3+ D.Cu2+
��4��4Fe(NO3)2�ĸߴ��Ƚᾧ����һ����ɫ�����Թ��壬�ʺ����ƴ�������������ĩ����ҵ���÷���м(��Fe��Fe2O3����������Ӧ������)��ȡFe(NO3)2����ķ�����ͼ��
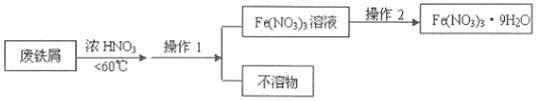
�ٵ�1����Ӧ���¶ȹ��ߣ�����������ֽ⣬Ũ�������ȷֽ�Ļ�ѧ����ʽΪ___��
�ڴ����������Ļ�ѧʽΪ___����д��Fe2O3�����ᷴӦ�����ӷ���ʽ___��
�۲���1������Ϊ___������2�IJ���Ϊ��___��___������ϴ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������нϹ㷺�Ŀ������ã���ṹ��ʽ����ͼ��ʾ��

���ڿ������������������ȷ����(����)
A������ʽΪC16H13O9
B��1 mol����������뺬8 mol NaOH����Һ��Ӧ
C����ʹ����KMnO4��Һ��ɫ��˵�����ӽṹ�к���̼̼˫��
D����Ũ��ˮ�ܷ����������͵ķ�Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com