
ЎҫМвДҝЎҝТ»¶ЁОВ¶ИПВЈ¬ПтИЭ»эОӘV LөДәгИЭГЬұХИЭЖчЦРНЁИл1 mol CH3Cl(g)әН1 mol H2O(g)Ј¬МеПөДЪН¬КұҙжФЪПВБРБҪёцЖҪәвЈә
·ҙУҰўЩЈәCH3Cl(g)Ј«H2O(g)![]() CH3OH(g)Ј«HCl(g) K1
CH3OH(g)Ј«HCl(g) K1
·ҙУҰўЪЈә2CH3OH(g)![]() (CH3)2O(g)Ј«H2O(g) K2
(CH3)2O(g)Ј«H2O(g) K2
·ҙУҰt minәуМеПөҙпөҪЖҪәвЈ¬ҙЛКұ(CH3)2O(g)өДОпЦКөДБҝОӘ9ЎБ10-3molЈ¬CH3Cl(g)өДЖҪәвЧӘ»ҜВКОӘ4.8%Ј¬»ШҙрПВБРОКМвЈә
(1)0Ў«t minДЪЈ¬CH3Cl(g)өД·ҙУҰЛЩВКОӘ___________ЎЈ
(2)·ҙУҰҙпөҪЖҪәвКұЈ¬CH3OH(g)өДОпЦКөДБҝОӘ___________ЎЈ
(3)јЖЛг·ҙУҰўЪөДЖҪәвіЈКэK2ЈҪ___________ЎЈ
(4)өұ·ҙУҰөҪҙпЖҪәвКұЈ¬ФЩПтМеПөДЪНЁИлТ»¶ЁБҝөДCH3OH(g)Ј¬ПВБРЛө·ЁХэИ·өДКЗ________(МоЧЦДё)ЎЈ
A.·ҙУҰўЩөДЖҪәвДжПтТЖ¶ҜЈ¬·ҙУҰўЪөДЖҪәвІ»·ўЙъТЖ¶Ҝ
B.ЖҪәвКұ·ҙУҰўЩЎў·ҙУҰўЪөД·ҙУҰЛЩВК¶јФцҙу
C.K1ФцҙуЈ¬K2јхРЎ
Ўҫҙр°ёЎҝ![]() molL-1min-1 0.03mol 9.61 B
molL-1min-1 0.03mol 9.61 B
ЎҫҪвОцЎҝ
Т»¶ЁОВ¶ИПВЈ¬ПтИЭ»эОӘV LөДәгИЭГЬұХИЭЖчЦРНЁИл1 mol CH3Cl(g)әН1 mol H2O(g)Ј¬МеПөДЪН¬КұҙжФЪПВБРБҪёцЖҪәвЈә
·ҙУҰўЩЈәCH3Cl(g)Ј«H2O(g)![]() CH3OH(g)Ј«HCl(g) K1
CH3OH(g)Ј«HCl(g) K1
·ҙУҰўЪЈә2CH3OH(g)![]() (CH3)2O(g)Ј«H2O(g) K2
(CH3)2O(g)Ј«H2O(g) K2
·ҙУҰҙпөҪЖҪәвКұТӘҝјВЗөҪCH3OH(g)өДЕЁ¶ИКЗБҪёц»ҜС§·ҙУҰЧЫәПәуөГөҪөДҪб№ыЎЈ
ЈЁ1Ј©·ҙУҰҝӘКјКұЈ¬CH3Cl(g)ОӘ1 molЈ¬t minәуCH3Cl(g)өДЖҪәвЧӘ»ҜВКОӘ4.8%Ј¬ЛщТФCH3Cl(g)өДОпЦКөДБҝұд»ҜБҝОӘ1mol![]() 4.8%=0.048molЈ¬»ҜС§·ҙУҰЛЩВК=
4.8%=0.048molЈ¬»ҜС§·ҙУҰЛЩВК=![]() =
=![]() =
=![]() molL-1min-1Ј¬№Кҙр°ёОӘ
molL-1min-1Ј¬№Кҙр°ёОӘ![]() molL-1min-1Ј»
molL-1min-1Ј»
ЈЁ2Ј©МеПөЦРБҪёц·ҙУҰН¬Кұ·ўЙъЈ¬УЙ·ҙУҰўЩCH3Cl(g)өДОпЦКөДБҝұд»ҜБҝОӘ0.048molЈ¬ҝЙЦӘ№ІЙъіЙCH3OH(g)өДОпЦКөДБҝОӘ0.048molЈ¬УЙ·ҙУҰўЪЦР(CH3)2O(g)өДОпЦКөДБҝОӘ9ЎБ10-3molЈ¬ҝЙЦӘПыәДөДCH3OH(g)өДОпЦКөДБҝОӘ9ЎБ10-3molЎБ2=0.018molЈ¬ЛщТФ·ҙУҰҙпөҪЖҪәвКұЈ¬CH3OH(g)өДОпЦКөДБҝОӘ0.048mol-0.018mol=0.03molЈ¬№Кҙр°ёОӘ0.03molЈ»
ЈЁ3Ј©·ҙУҰўЩҙпөҪЖҪәвәуГ»УРІОјУ·ҙУҰөДH2O(g)өДОпЦКөДБҝОӘ1mol-0.048mol=0.952molЈ¬·ҙУҰўЪЙъіЙөДH2OЈЁgЈ©өДОпЦКөДБҝОӘ9ЎБ10-3molЈ¬ЛщТФЖҪәвКұИЭЖчДЪH2OЈЁgЈ©өДОпЦКөДБҝЕЁ¶ИОӘ![]() =
=![]() molL-1Ј¬(CH3)2O(g)өДОпЦКөДБҝЕЁ¶ИОӘ
molL-1Ј¬(CH3)2O(g)өДОпЦКөДБҝЕЁ¶ИОӘ![]() =
=![]() molL-1Ј¬УЙЈЁ2Ј©ҝЙЦӘЖҪәвКұCH3OH(g)өДОпЦКөДБҝЕЁ¶ИОӘ
molL-1Ј¬УЙЈЁ2Ј©ҝЙЦӘЖҪәвКұCH3OH(g)өДОпЦКөДБҝЕЁ¶ИОӘ![]() =
=![]() molL-1Ј¬ЖҪәвіЈКэK2ЈҪ
molL-1Ј¬ЖҪәвіЈКэK2ЈҪ![]() =9.61Ј¬№Кҙр°ёОӘ9.61Ј»
=9.61Ј¬№Кҙр°ёОӘ9.61Ј»
ЈЁ4Ј©өұ·ҙУҰөҪҙпЖҪәвКұЈ¬ФЩПтМеПөДЪНЁИлТ»¶ЁБҝөДCH3OH(g)Ј¬CH3OH(g)ОпЦКөДБҝЕЁ¶ИФцҙуЈ¬·ҙУҰўЩөДЖҪәвДжПтТЖ¶ҜЈ¬·ҙУҰўЪөДЖҪәвХэПтТЖ¶ҜЈ¬ЖҪәвКұ·ҙУҰўЩЎў·ҙУҰўЪөД·ҙУҰЛЩВК¶јФцҙуЈ¬ОВ¶ИІ»ұдКұЈ¬·ҙУҰөДЖҪәвіЈКэ¶јІ»ёДұдЈ¬№Кҙр°ёСЎBЎЈ



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ№ӨТө·ПЖшЎўЖыіөОІЖшЕЕ·ЕіцөДNOxЎўSO2өИЈ¬КЗРОіЙОнцІөДЦчТӘОпЦКЈ¬ЖдЧЫәПЦОАнКЗөұЗ°ЦШТӘөДСРҫҝҝОМвЎЈ
ЈЁ1Ј©ТСЦӘЈәўЩCOИјЙХИИөДЎчH1=Јӯ283.0kJЎӨmol-lЈ¬ўЪN2(g)+O2(g) ![]() 2NO(g) ЎчH2=+180.5kJЎӨmol-1Ј¬ЖыіөОІЖшЦРөДNO(g)әНCO(g)ФЪТ»¶ЁОВ¶ИәНҙЯ»ҜјБМхјюПВҝЙ·ўЙъИзПВ·ҙУҰЈә2NO(g)+2CO(g)
2NO(g) ЎчH2=+180.5kJЎӨmol-1Ј¬ЖыіөОІЖшЦРөДNO(g)әНCO(g)ФЪТ»¶ЁОВ¶ИәНҙЯ»ҜјБМхјюПВҝЙ·ўЙъИзПВ·ҙУҰЈә2NO(g)+2CO(g) ![]() N2(g)+2CO2(g)Ј» ЎчH=___ЎЈ
N2(g)+2CO2(g)Ј» ЎчH=___ЎЈ
ЈЁ2Ј©Ҫ«0Ј®20mol NOәН0Ј®10molCOідИлТ»ёцИЭ»эәг¶ЁОӘ1LөДГЬұХИЭЖчЦР·ўЙъЙПКц·ҙУҰЈ¬·ҙУҰ№эіМЦРІҝ·ЦОпЦКөДЕЁ¶Иұд»ҜИзПВНјЛщКҫЈ®
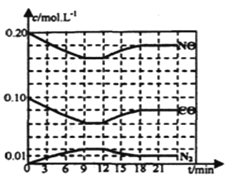
ўЩёГ·ҙУҰөЪТ»ҙОҙпөҪЖҪәвКұөДЖҪәвіЈКэОӘ________ЎЈ
ўЪөЪ12minКұёДұдөДМхјюКЗ________ЎЈ
ўЫФЪөЪ24minКұЈ¬ИфұЈіЦОВ¶ИІ»ұдЈ¬ФЩПтИЭЖчЦРідИлCOәНN2ёч0Ј®060molЈ¬ЖҪәвҪ«________ТЖ¶Ҝ(МоЎ°ХэПтЎұЎўЎ°ДжПтЎұ»тЎ°І»Ўұ)Ј®
(3)SNCR-SCRНСПхјјКхКЗТ»ЦЦРВРНөДіэИҘСМЖшЦРөӘСх»ҜОпөДНСПхјјКхЈ¬Т»°гІЙУГ°ұЖш»тДтЛШЎЈ
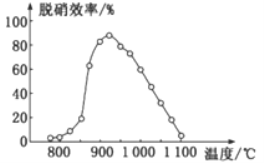
ўЩSNCRНСПхјјКхЦРЈәФЪҙЯ»ҜјБЧчУГПВУГNH3Чч»№ФӯјБ»№ФӯNOЈ¬ЖдЦчТӘ·ҙУҰОӘЈә4NH3(g)+4NO(g)+O2(g)=4N2(g)+6H2O(g)Ј¬ЎчH<0ЎЈМеПөОВ¶ИЦұҪУУ°ПмSNCRјјКхөДНСПхР§ВКЈ¬ИзНјЛщКҫЎЈөұМеПөОВ¶ИФјОӘ925ЎжКұЈ¬SNCRНСПхР§ВКЧоёЯЈ¬ЖдҝЙДЬөДФӯТтКЗ________ЎЈ
ўЪSCRНСПхјјКхЦРФтУГДтЛШ[CO(NH2)2]Чч»№ФӯјБ»№ФӯNO2өД»ҜС§·ҪіМКҪОӘ____________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПЦУРТ»¶ЁБҝә¬УРNa2OФУЦКөДNa2O2КФСщЈ¬УГПВНјөДКөСйЧ°ЦГІв¶ЁNa2O2КФСщөДҙҝ¶ИЎЈ(ҝЙ№©СЎУГөД·ҙУҰОпЦ»УРCaCO3№ММеЎў6 molЎӨLЈӯ1СОЛбЎў6 molЎӨLЈӯ1БтЛбәНХфБуЛ®)

»ШҙрПВБРОКМвЈә
ЈЁ1Ј©°ҙИзНјЛщКҫөДЧ°ЦГБ¬ҪУНкТЗЖчЈ¬ФЪјУТ©Ж·Ц®З°УҰёГЧцөДІЩЧчКЗ______________
ЈЁ2Ј©Ч°ЦГAЦРТәМеКФјБСЎУГ_____________________________Ј¬
ЈЁ3Ј©Ч°ЦГBөДЧчУГКЗ___________________________________Ј¬Ч°ЦГCөДЧчУГКЗ____________________________________Ј¬Ч°ЦГEЦРјоКҜ»ТөДЧчУГКЗ____________________________________________Ј¬
ЈЁ4Ј©Ч°ЦГDЦР·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪКЗ______________________________________
ЈЁ5Ј©ИфҝӘКјКұІвөГСщЖ·өДЦКБҝОӘ2.0 gЈ¬·ҙУҰҪбКшәуІвөГЖшМеМе»эОӘ224 mL(ұкЧјЧҙҝц)Ј¬ФтNa2O2КФСщөДҙҝ¶ИОӘ________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБРЛө·ЁХэИ·өДКЗ
A.°ҙПөНіГьГы·ЁЈ¬»ҜәПОп![]() өДГыіЖКЗ2Ј¬3Ј¬5Ј¬5ЈӯЛДјЧ»щЈӯ4Ј¬4Јӯ¶юТТ»щјәНй
өДГыіЖКЗ2Ј¬3Ј¬5Ј¬5ЈӯЛДјЧ»щЈӯ4Ј¬4Јӯ¶юТТ»щјәНй
B.өИОпЦКөДБҝөДұҪәНұҪјЧЛбНкИ«ИјЙХПыәДСхЖшөДБҝІ»ПаөИ
C.ұҪәНјЧұҪ»ҘОӘН¬ПөОпЈ¬ҫщДЬК№KMnO4ЛбРФИЬТәНКЙ«
D.Ҫб№№Ж¬¶О өДёЯҫЫОпЈ¬ЖдөҘМеКЗұҪ·УәНјЧИ©
өДёЯҫЫОпЈ¬ЖдөҘМеКЗұҪ·УәНјЧИ©
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДЙГЧМјЛбёЖ№г·әУҰУГУЪПрҪәЎўЛЬБПЎўФмЦҪЎў»ҜС§ҪЁІДЎўУНД«ЎўНҝБПЎўГЬ·вҪәУлҪәХіјБөИРРТөЎЈФЪЕЁCaCl2ИЬТәЦРНЁИлNH3әНCO2Ј¬ҝЙТФЦЖөГДЙГЧј¶МјЛбёЖЎЈДіРЈС§ЙъКөСйРЎЧйЙијЖПВНјЛщКҫЧ°ЦГЈ¬ЦЖИЎёГІъЖ·ЎЈDЦРЧ°УРХәПЎБтЛбөДНСЦ¬ГЮЈ¬НјЦРјРіЦЧ°ЦГТСВФИҘЎЈ
ўсЈ®ҝЙСЎУГөДТ©Ж·УРЈә
aЈ®КҜ»ТКҜЈ»bЈ®ұҘәНВИ»ҜёЖИЬТәЈ»cЈ®6 mol/LСОЛбЈ»dЈ®ВИ»Ҝп§Ј»eЈ®ЗвСх»ҜёЖ
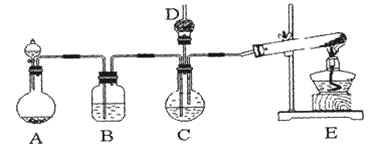
ЈЁ1Ј©AЦРЦЖұёЖшМеКұЈ¬ЛщРиТ©Ж·КЗЈЁСЎМоЧЦДёРтәЕЈ©______________Ј»
ЈЁ2Ј©BЦРКўУРұҘәНМјЛбЗвДЖИЬТәЈ¬ЖдЧчУГКЗ______________________________Ј»
ЈЁ3Ј©РҙіцЦЖИЎ°ұЖшөД»ҜС§·ҪіМКҪ__________________________________Ј»
ЈЁ4Ј©ФЪКөСй№эіМЦРЈ¬ПтCЦРНЁИлЖшМеКЗУРПИәуЛіРтөДЈ¬УҰПИНЁИлЖшМеөД»ҜС§КҪ______________Ј»
ЈЁ5Ј©јмСйDіцҝЪҙҰКЗ·сУР°ұЖшТЭіцөД·Ҫ·ЁКЗ__________________________Ј»
ЈЁ6Ј©РҙіцЦЖДЙГЧј¶МјЛбёЖөД»ҜС§·ҪіМКҪ______________________________ЎЈ
ЈЁ7Ј©ИфКөСй№эіМЦРУР°ұЖшТЭіцЈ¬УҰСЎУГПВБР_____________Ч°ЦГ»ШКХЈЁМоҙъәЕЈ©ЎЈ
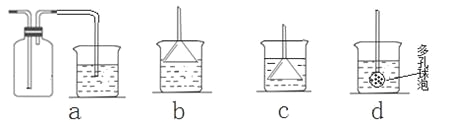
ўтЈ®ҫӯ·ЦОцФЪЙПКцВИ»Ҝп§СщЖ·ЦРә¬УРФУЦКМјЛбЗвДЖЎЈОӘБЛІв¶ЁВИ»Ҝп§өДЦКБҝ·ЦКэЈ¬ёГС§ЙъКөСйРЎЧйУЦЙијЖБЛИзПВКөСйБчіМЈә
КФ»ШҙрЈә
ЈЁ1Ј©ЛщјУКФјБAөД»ҜС§КҪОӘ______________________________________Ј»
ЈЁ2Ј©BІЩЧч·Ҫ·ЁКЗ_______________________________________________Ј»
ЈЁ3Ј©СщЖ·ЦРВИ»Ҝп§өДЦКБҝ·ЦКэОӘ___________________________________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝТСЦӘИзПВБҪёцИИ»ҜС§·ҙУҰ
![]() (l)Ј«H2(g)Ўъ
(l)Ј«H2(g)Ўъ![]() (l)
(l)![]() HЈҫ0 ўЩ
HЈҫ0 ўЩ
![]() (l)Ј«2H2(g)Ўъ
(l)Ј«2H2(g)Ўъ![]() (l)
(l)![]() HЈј0 ўЪ
HЈј0 ўЪ
ПВБРЛө·ЁІ»ХэИ·өДКЗ
A.·ҙУҰўЩЎўўЪ¶јКфУЪјУіЙ·ҙУҰ
B.lЈ¬3Т»»·јә¶юП©ұИұҪОИ¶Ё
C.·ҙУҰўЩЎўўЪөДИИР§УҰЛөГчұҪ»·ЦРә¬УРөДІўІ»КЗМјМјЛ«јь
D.·ҙУҰўЩЎўўЪЦРөДЛщУРУР»ъОпҫщҝЙК№деЛ®НКЙ«Ј¬ө«НКЙ«ФӯАнІ»НкИ«ПаН¬
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝМјКЗРОіЙ»ҜәПОпЦЦАаЧо¶аөДФӘЛШЈ¬ЖдөҘЦКј°РОіЙөД»ҜәПОпКЗИЛАаЙъІъЙъ»оөДЦчТӘДЬФҙОпЦКЎЈ
(1)УР»ъОпMҫӯ№эМ«Сф№в№вХХҝЙЧӘ»ҜіЙОпЦКNЈ¬ЖдДЬБҝұд»ҜИзНјЛщКҫЎЈФтMЎўNПаұИЈ¬ҪПОИ¶ЁөДКЗ________(МоЎ°MЎұ»тЎ°NЎұ)ЎЈ

(2)ТСЦӘЈә
C(s)Ј«H2O(l)ЈҪCO(g)Ј«H2(g) ЎчH1ЈҪa kJЎӨmol-1
2CO(g)Ј«O2(g)ЈҪ2CO2(g) ЎчH2ЈҪb kJЎӨmol-1
2H2(g)Ј«O2(g)ЈҪ2H2O(l) ЎчH3ЈҪc kJЎӨmol-1
ФтC(s)Ј«O2(g)ЈҪCO2(g) ЎчHЈҪ______(УГaЎўbЎўcұнКҫ)kJЎӨmol-1ЎЈ
(3)ёщҫЭјьДЬКэҫЭ№АЛгCH4(g)Ј«4F2(g)ЈҪCF4(g)Ј«4HF(g)өД·ҙУҰИИЎчHЈҪ_________ЎЈ
»ҜС§јь | C-H | C-F | H-F | F-F |
јьДЬ(KJmol-1) | 414 | 489 | 565 | 155 |

(4)ФЪТ»әгИЭөДГЬұХИЭЖчЦРЈ¬јУИл1 mol CO(g)Ўў2 mol H2O(g)Ј¬·ўЙъ·ҙУҰCO(g)Ј«H2O(g)![]() H2(g)Ј«CO2(g) ЎчHЈ¬COөДЖҪәвЧӘ»ҜВКЛжОВ¶ИөДұд»ҜИзНјЛщКҫЎЈ
H2(g)Ј«CO2(g) ЎчHЈ¬COөДЖҪәвЧӘ»ҜВКЛжОВ¶ИөДұд»ҜИзНјЛщКҫЎЈ

ўЩёГ·ҙУҰөДЎчH________(МоЎ°<Ўұ»тЎ°>Ўұ)0ЎЈ
ўЪФЪМе»эІ»ұдКұЈ¬ТӘФцҙуёГ·ҙУҰөДХэ·ҙУҰЛЩВКҝЙІЙИЎөДҙлК©КЗ_________(ИОРҙТ»Мх)ЎЈ
ўЫAөгКұёГ·ҙУҰөДЖҪәвіЈКэОӘ___________(ҫ«И·өҪ0.01)ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝУРПВБРИэЦЦУР»ъОпЈ¬КөПЦЛьГЗЦ®јдПа»ҘЧӘ»ҜЛщСЎКФјБЈЁҫщЧгБҝЈ©ХэИ·өДКЗЈЁ Ј©
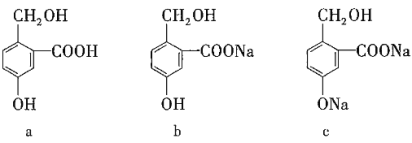
СЎПо | aЧӘ»ҜОӘb | aЧӘ»ҜОӘc | cЧӘ»ҜОӘb |
A | NaOH | Na | CO2 |
B | Na2CO3 | NaOH | HCl |
C | NaHCO3 | NaOH | CO2 |
D | NaHCO3 | NaCl | HCl |
A.AB.BC.CD.D
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝёщҫЭПВБРКөСйДЬөГіцПаУҰҪбВЫөДКЗ
СЎПо | КөСй | ҪбВЫ |
A | іЈОВПВ,ІвөГ0.1mol/LNaAИЬТәөДpHРЎУЪ0.1mol/L Na2CO3ИЬТәөДpH | ЛбРФ:HA>H2CO3 |
B | Птә¬УРөн·ЫөДFeI2ИЬТәЦРјУИлЧгБҝдеЛ®,ИЬТәұдА¶Й« | »№ФӯРФ:I->Fe2+ |
C | ПтұҘәНFeSO4ИЬТәЦРјУИлCuS№ММе,ІвөГИЬТәЦРc(Fe2+)І»ұд | Ksp(CuS)<Ksp(FeS) |
D | Пт°ұЛ®ЦРөОјУЙЩБҝAgNO3ИЬТәЈ¬ОЮіБөнЙъіЙ | Ag+УлNH3ЎӨH2OДЬҙуБҝ№Іҙж |
A. A B. B C. C D. D
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com