
����Ŀ���л���I(����ʽΪC19H20O4)���ڷ����������ʣ���һ�ֵ��������ϳ�·�����£�
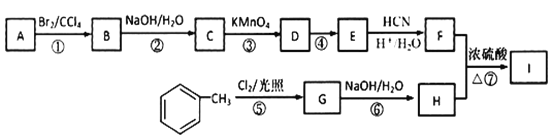
��֪����A����֬�������˴Ź���������ʾ��2��壬�����Ϊ3��1���������ܶ�����ͬ������H2��28����D����ʽΪC4H8O3��E����ʽΪC4H6O2����ʹ��ˮ��ɫ��
��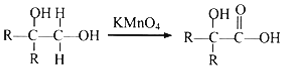 ����R������
����R������
��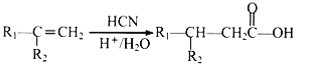 ����R1��R2��������
����R1��R2��������
�ش��������⣺
(1)A�Ľṹ��ʽΪ___________________��
(2)D�й����ŵ�������______________________��
(3)��Ӧ�ڵĻ�ѧ����ʽΪ______________��
(4)E��ͬϵ��K��E��һ��̼ԭ�ӣ�K�ж�����״ͬ���칹�壬�����ܷ���������Ӧ����ˮ�����_______�֡�
(5)��Ӧ�١�������ȡ����Ӧ����__________(�����)��
(6)��Ӧ�ߵĻ�ѧ����ʽΪ_________________��
(7)���������ϳ�·�ߣ��� 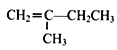 Ϊԭ��(���Լ���ѡ)����4����Ӧ�Ʊ��ɽ�������
Ϊԭ��(���Լ���ѡ)����4����Ӧ�Ʊ��ɽ�������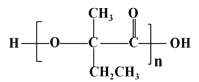 _____________��
_____________��
���𰸡�CH3C(CH3)=CH2 �ǻ����Ȼ� CH3CBr(CH3)=CH2Br+2NaOH![]() CH3C(OH)(CH3)CH2OH+2NaBr 8 �ڢݢޢ�
CH3C(OH)(CH3)CH2OH+2NaBr 8 �ڢݢޢ� 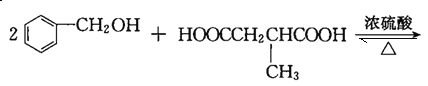
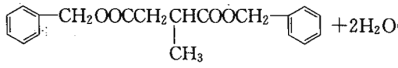
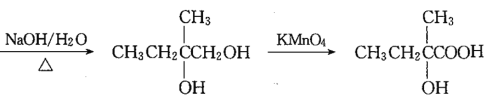
![]()
��������
������֪��M(A)=28��2=56g��mo1-1,A����֬����,A�ķ���ʽΪC4H8,A�ĺ˴Ź���������ʾ��2����������Ϊ3:1,A�Ľṹ��ʽΪCH3C(CH3)=CH2�����ݸ�����Ӧ����![]() ��֪��B�Ľṹ��ʽΪCH3CBr(CH3)=CH2Br,C�Ľṹ��ʽΪCH3C(OH)(CH3)CH2OH,���
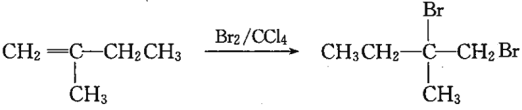
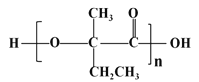
��֪��B�Ľṹ��ʽΪCH3CBr(CH3)=CH2Br,C�Ľṹ��ʽΪCH3C(OH)(CH3)CH2OH,���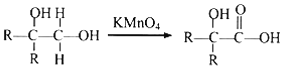 ��֪DΪCH3C(OH)(CH3)COOH,��E����ʽΪC4H6O2����ʹ��ˮ��ɫ����֪EΪCH2=C(CH3)COOH��������Ϣ
��֪DΪCH3C(OH)(CH3)COOH,��E����ʽΪC4H6O2����ʹ��ˮ��ɫ����֪EΪCH2=C(CH3)COOH��������Ϣ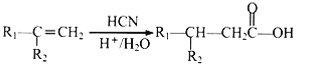 ��֪FΪHOOCCH2CH(CH3)COOH���ɿ�ͼ
��֪FΪHOOCCH2CH(CH3)COOH���ɿ�ͼ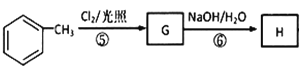 ���ݷ�Ӧ�������ƿ�֪GΪ
���ݷ�Ӧ�������ƿ�֪GΪ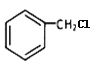 ��HΪ
��HΪ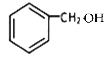 ��
��
(1)������֪��M(A)=28��2=56g��mo1-1,A����֬����,�á��̷���,56��12=4��8,A�ķ���ʽΪC4H8,A�ĺ˴Ź���������ʾ��2����������Ϊ3:1,��A�Ľṹ��ʽΪCH3C(CH3)=CH2;�𰸣�CH3C(CH3)=CH2��
(2)������������DΪCH3C(OH)(CH3)COOH�����к��й����ŵ��������ǻ����Ȼ����𰸣��ǻ����Ȼ���
(3)��Ӧ�� ����BΪCH3CBr(CH3)=CH2Br,CΪCH/span>3C(OH)(CH3)CH2OH������ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪCH3CBr(CH3)=CH2Br+2NaOH
����BΪCH3CBr(CH3)=CH2Br,CΪCH/span>3C(OH)(CH3)CH2OH������ˮ�ⷴӦ����Ӧ�Ļ�ѧ����ʽΪCH3CBr(CH3)=CH2Br+2NaOH![]() CH3C(OH)(CH3)CH2OH+2NaBr���𰸣�CH3CBr(CH3)=CH2Br+2NaOH
CH3C(OH)(CH3)CH2OH+2NaBr���𰸣�CH3CBr(CH3)=CH2Br+2NaOH![]() CH3C(OH)(CH3)CH2OH+2NaBr��
CH3C(OH)(CH3)CH2OH+2NaBr��
(4)��EΪCH2=C(CH3)COOH��E�ķ���ʽΪC4H6O2,E��ͬϵ��K��E��-��̼ԭ��,K�ķ���ʽΪC5H8O2��K�IJ����Ͷ�Ϊ2,K��ͬ���칹���ܷ���������Ӧ����ˮ�⣬�������Oԭ�Ӹ���,K��ͬ���칹��Ϊ����ij����������������״ͬ���칹����HOOOCH=CHCH2CH3(�ƶ�˫����3��)��HCOOC(CH3)=CHCH3(�ƶ�˫����-CH3��5��)����8�֣��𰸣�8��
(5)��Ӧ��![]() �ߵķ�Ӧ��������Ϊ�ӳɷ�Ӧ��ȡ����Ӧ(��ˮ�ⷴӦ)��������Ӧ����ȥ��Ӧ,ȡ����Ӧ��ȡ����Ӧ(��ˮ�ⷴӦ)��ȡ����Ӧ��������Ӧ),����ȡ����Ӧ���Ǣڢݢޢߡ��𰸣��ڢݢޢߡ�
�ߵķ�Ӧ��������Ϊ�ӳɷ�Ӧ��ȡ����Ӧ(��ˮ�ⷴӦ)��������Ӧ����ȥ��Ӧ,ȡ����Ӧ��ȡ����Ӧ(��ˮ�ⷴӦ)��ȡ����Ӧ��������Ӧ),����ȡ����Ӧ���Ǣڢݢޢߡ��𰸣��ڢݢޢߡ�
(6)�ɿ�ͼ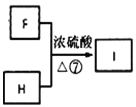 ����������������֪FΪHOOCCH2CH(CH3)COOH��HΪ
����������������֪FΪHOOCCH2CH(CH3)COOH��HΪ ��I����ʽΪC19H20O4����Ӧ�ߵĻ�ѧ����ʽΪHOOCCH2CH(CH3)COOH+2
��I����ʽΪC19H20O4����Ӧ�ߵĻ�ѧ����ʽΪHOOCCH2CH(CH3)COOH+2
![]()
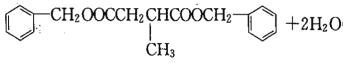 ���𰸣�HOOCCH2CH(CH3)COOH+2
���𰸣�HOOCCH2CH(CH3)COOH+2
![]()
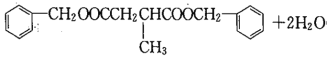 ��
��
(7)��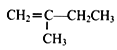 Ϊԭ�ϣ���4����Ӧ�Ʊ��ɽ�������
Ϊԭ�ϣ���4����Ӧ�Ʊ��ɽ������� �ĺϳ�·��Ϊ��
�ĺϳ�·��Ϊ��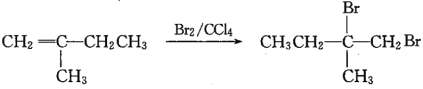
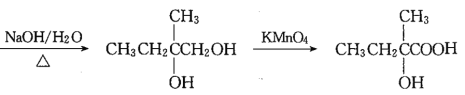
![]()
 ��
��


 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪1 g������ȫȼ������ˮ����ʱ�ų�����121 kJ����������1 mol O=O����ȫ����ʱ��������496 kJ��ˮ������1mol H-O���γ�ʱ�ų�����463 kJ����������1mol H-H������ʱ��������Ϊ
A.920 kJB.557 kJC.436 kJD.188 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪������ A ������Ԫ����ɣ�ij�о�С�鰴��ͼ����̽������ɣ����� D ��һ����ʹƷ����Һ��ɫ�����塣
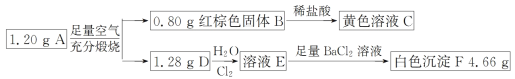
��ش��������⣺
(1)������ A �����Ԫ����______��(��Ԫ�ط���)
(2)д�� A��B+D �Ļ�ѧ����ʽ_________��
(3)д�� D��E �����ӷ���ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����д��м��ܷ�����ȥ��Ӧ�����ܱ�����Ϊͪ���ǣ� ��
�� ��
��![]() ��CH3OH
��CH3OH
�� ��
�� ��
��
A. �٢ܢ� B. �ڢ� C. �ڢ� D. �٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ѧ��ѧ���õ��Լ�����Ҫ���ڷ�������������ҩ�ȡ�ʵ����ģ�ҵ�������̿��Ʊ�������ص��������£�
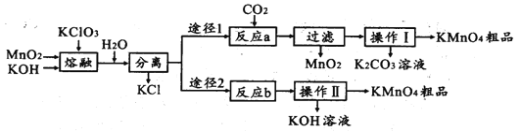
��1��ʵ�������ڶ������̡��������ء������ʱӦѡ��____________________________
a.��ͨ�������� b.ʯӢ���� c.�մ����� d.������
��2����һ������ʱ����K2MnO4�Ļ�ѧ����ʽ��______________________________________
��3���������и���KMnO4��K2CO3��������____________ (������)�ϵIJ��죬����Ũ���ᾧ�����ȹ��˵õ�KMnO4�����ȹ��˵�ԭ����______________________________________
��4����Ӧb�ǵ�ⷨ�Ʊ�KMnO4����װ����ͼ��ʾ��a��____________��(����������������)���м�����ӽ���Ĥ��_____ (����������������)���ӽ���Ĥ�������ĵ缫��ӦʽΪ____________
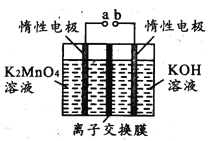
��5��ȡ��Ӧb��ĵ��Һ���������ܽ����0.1000mol ��L��1FeSO4����Һ�ζ����յ�ʱ������250.0mL����Һ���������Һ�е�KMnO4��������_________g��
��6������;�����Ʊ�������;��1��;��2���۲���֮��Ϊ_________
��7��KMnO4ϡ��Һ��һ�ֳ��õ���������������ԭ��������������ͬ����________(����)��
a.˫��ˮ b.84��Һ(NaClO��Һ) c.75���ƾ� d.����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A. ����ˮ�����������顢�����顢�Ҵ�����Һ̬�л���
B. ̼ԭ����С�ڻ����6�ĵ�ϩ������HBr�ӳɷ�Ӧ�IJ���ֻ��1�ֽṹ�����������ĵ�ϩ����3��
C. �������顢��ϩ����Ȳ������̼̼���ļ����ֱ�Ϊa��b��c��d����![]() >d
>d
D. ������������ȫȼ�գ������������Ǽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽��һ�廷����(![]() )��NaOH�Ĵ���Һ���ȷ�������ˮ�ⷴӦ������ȥ��Ӧ��������λͬѧ�ֱ��������ʵ�鷽���������ж���ȷ����
)��NaOH�Ĵ���Һ���ȷ�������ˮ�ⷴӦ������ȥ��Ӧ��������λͬѧ�ֱ��������ʵ�鷽���������ж���ȷ����
A. ��Ӧ���Һ�е���ϡ�����к�NaOH��Һ��Ȼ�����AgNO3��Һ������dz��ɫ�������ɣ����֤����������ȥ��Ӧ��
B. ��Ӧ���Һ�е�����ˮ������Һ��ɫ�ܿ���ȥ�����֤����������ȥ��Ӧ��
C. ��Ӧ���Һ�е�������KMnO4��Һ������Һ��ɫ��dz�����֤����������ȥ��Ӧ
D. ��Ӧ���Һ���ȼ������ữ���ټ�����ˮ�������Һ��ɫ�ܿ���ȥ����֤����������ȥ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵������ȷ����(����)
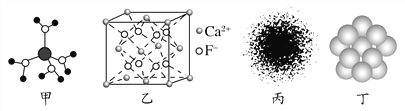
A. ˮ��ͭ���ӵ�ģ����ͼ����ʾ��1��ˮ��ͭ��������4����λ��
B. CaF2����ľ�����ͼ����ʾ��ÿ��CaF2����ƽ��ռ��4��Ca2��
C. Hԭ�ӵĵ�����ͼ��ͼ����ʾ��Hԭ�Ӻ�������������ԭ�Ӻ˸����˶�
D. ����Cu��Cuԭ�Ӷѻ�ģ����ͼ����ʾ��Ϊ���ܶѻ���ÿ��Cuԭ�ӵ���λ����Ϊ12
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��100 mL 0.3 mol��L��1 Na2SO4��Һ��50 mL 0.2 mol��L��1 Al2(SO4)3��Һ��Ϻ���Һ��SO42-�����ʵ���Ũ��Ϊ(����)
A. 0.20 mol��L��1B. 0.25 mol��L��1
C. 0.40 mol��L��1D. 0.50 mol��L��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com