
����Ŀ����1���ֽ�200mL0.30mol/L��������50mL0.80mol/LCaCl2��Һ���(��Ϻ�����仯���Բ���)��������Һ��Cl�������ʵ���Ũ����___��
��2����20.0g�������ƹ�������ˮ���100mL��Һ�����ܶ�Ϊ1.25g��mL��1������Һ���������Ƶ����ʵ���Ũ��Ϊ___����������Ϊ___���Ӹ���Һ��ȡ��10 mL�������ˮϡ�͵�100 mL��ϡ�ͺ���Һ���������Ƶ����ʵ���Ũ��Ϊ___����һ�������ԭ��Һ��ϡ�ͺ����Һ��1��4������Ȼ�ϣ����Ի��ʱ��Һ����仯�������û����Һ���������Ƶ����ʵ���Ũ��Ϊ___��
��3�����廯����A����ʽ�ɱ�ʾΪOxFy����֪ͬ��ͬѹ��10mLA���ȷֽ�����15mLO2��10mLF2����A�Ļ�ѧʽΪ___���ƶϵ�����Ϊ___��
���𰸡�0.56mol/L 5mol/L 16% 0.5mol/L 1.4mol/L O3F2 ͬ��ͬѹ�£����������ȵ������ʵ���֮���ֵ��ڻ�ѧ����ʽ������֮��
��������
��1����200mL0.30mol/L��������50mL0.80mol/LCaCl2��Һ���(��Ϻ�����仯���Բ���)��������Һ��Cl�������ʵ���Ũ����![]() ����Ϊ��0.56mol/L��
������0.56mol/L��
��2����20.0g�������ƹ�������ˮ���100mL��Һ�����ܶ�Ϊ1.25g��mL��1������Һ���������Ƶ����ʵ���Ũ��Ϊ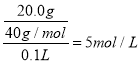 ����Ϊ��5mol/L��
������5mol/L��
��������Ϊ![]() ����Ϊ��16%��
������16%��
�Ӹ���Һ��ȡ��10 mL�������ˮϡ�͵�100 mL��ϡ�ͺ���Һ���������Ƶ����ʵ���Ũ��Ϊ![]() ����Ϊ��0.5mol/L��
������0.5mol/L��
��һ�������ԭ��Һ��ϡ�ͺ����Һ��1��4������Ȼ�ϣ����Ի��ʱ��Һ����仯�������û����Һ���������Ƶ����ʵ���Ũ��Ϊ![]() ����Ϊ��1.4mol/L��
������1.4mol/L��
��3�����廯����A����ʽ�ɱ�ʾΪOxFy����֪ͬ��ͬѹ��10mLA���ȷֽ�����15mLO2��10mLF2��
2OxFy = xO2 + yF2
2mL xmL ymL
10mL 15mL 10mL
��ã�x=3 ��y=2
��A�Ļ�ѧʽΪO3F2����Ϊ��O3F2��
�ƶϵ�����Ϊ��ͬ��ͬѹ�£����������ȵ������ʵ���֮���ֵ��ڻ�ѧ����ʽ������֮�ȡ�
��Ϊ��ͬ��ͬѹ�£����������ȵ������ʵ���֮���ֵ��ڻ�ѧ����ʽ������֮�ȡ�


 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������������һ����Ҫ�Ļ���ԭ�ϡ�
(1)�Լ���Ϊԭ�ϣ������ַ����ϳɼ״���
����������CH4(g)+![]() O2(g)
O2(g)![]() CO(g)+2H2(g) ��H1=-35.4kJ/mol
CO(g)+2H2(g) ��H1=-35.4kJ/mol
��CO(g)��2H2(g)![]() CH3OH(g) ��H2 =-90.1kJ/mol
CH3OH(g) ��H2 =-90.1kJ/mol
������ ��2CH4(g)��O2(g)![]() 2CH3OH(g) ��H3 =______kJ/mol
2CH3OH(g) ��H3 =______kJ/mol
(2)���ܱ������г���2molCH4 (g) ��1molO2 (g)���ڲ�ͬ�����·�Ӧ��2CH4(g)+O2(g)![]() 2CH3OH(g)��ʵ����ƽ��ʱ�״������ʵ������¶ȡ�ѹǿ�ı仯��ͼ��ʾ��
2CH3OH(g)��ʵ����ƽ��ʱ�״������ʵ������¶ȡ�ѹǿ�ı仯��ͼ��ʾ��

��P1ʱ�����¶ȣ�n(CH3OH)_______________������������������С����������������
��E��F��N���Ӧ�Ļ�ѧ��Ӧ�����ɴ�С��˳��Ϊ____________����V(E)��V(F)��V(N)��ʾ����
�����������CH4ƽ��ת���ʵĴ�ʩ��_______________������ţ�
a.ѡ���Ч���� b.����![]() Ͷ�ϱ� c.��ʱ�������
Ͷ�ϱ� c.��ʱ�������
����F��n (CH3OH)=1mol����ѹǿΪ2.5MPa,��T0ʱF���÷�ѹǿ����Ũ�ȱ�ʾ��ƽ�ⳣ��Kp=_____________________ ��
(3)ʹ�����ʹ������з�Ӧ2CH4(g)+O2 (g)![]() 2CH3OH(g)�����¶�����CH3OH�IJ�����ͼ��ʾ��
2CH3OH(g)�����¶�����CH3OH�IJ�����ͼ��ʾ��

��CH3OH�IJ�����T1��T2ʱ�ܿ������ԭ����______________��
��T2��CH3OH���ʽ��͵�ԭ�������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��E�Ǻϳ�ijҩ����м��壬��һ�ֺϳ�·����ͼ��

��1��A�к��������ŵ�������___��
��2��A��B�ķ�Ӧ�������Լ���___��
��3��D��E�ķ�Ӧ������___��
��4��д��B��C�Ļ�ѧ����ʽ___��
��5��E�ķ���ʽΪ___��
��6��B�Ļ��϶��������___��(�����������칹)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ���BCl3������Ҫ�����뵼���IJ���Դ���л��ϳɴ����������ڸߴ�����л������ȡ��ij��ȤС������������Ϊԭ�ϣ���������װ�ã�����װ�ÿ��ظ�ʹ�ã��Ʊ�BCl3��

��֪����BCl3�ķе�Ϊ12.5�棬�۵�Ϊ-107.3�棻��ˮ���ҷ�Ӧ��������������2B+6HCl ![]() 2BCl3+3H2�������������������ƣ�Ҳ��������������Һ��Ӧ��
2BCl3+3H2�������������������ƣ�Ҳ��������������Һ��Ӧ��
��ش��������⣺
��1��Aװ�ÿ�������ع�����Ũ���ᷴӦ����������Ӧ�Ļ�ѧ����ʽΪ___________��
��2��װ�ô����ҵĽӿ�����˳��Ϊa��___________________��j��
��3��װ��E�е��Լ�Ϊ___________�������ȥEװ�ã����ܵĺ����____________��
��4��Dװ���з�����Ӧǰ��ͨ��һ��ʱ����������ž�װ���еĿ�������ȱ�ٴ˲��裬����ɵĽ����_____��
��5�����Ȼ�����ˮ�ܾ��ҷ�Ӧ�������ᣨH3BO3���Ͱ�����д���÷�Ӧ�Ļ�ѧ����ʽ________������Ҳ���õ��������Ʊ��������ҵ�������������ԭ����ͼ��ʾ��

�������ĵ缫��Ӧʽ__________________��������Ʒ�ҿɵõ�H3BO3��ԭ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ӧ����Ϣ���л��ϳ��о�����Ҫ���ã�������������Ϣ�ش����⣺
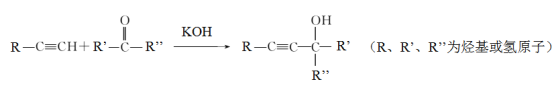
(1)�÷�Ӧ����Ϊ_______________
(2)���������ܷ���������Ӧ����______
A.![]() ��
��![]()
B.![]() ��HCHO
��HCHO
C.![]() ��
��![]()
(3)![]() ��
��![]() �������������ܹ��ϳɷ���ʽΪC8H14O2�����ʣ������ʵĽṹ��ʽΪ________________
�������������ܹ��ϳɷ���ʽΪC8H14O2�����ʣ������ʵĽṹ��ʽΪ________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��������ָ�뷢��ƫת��ͬʱA���������٣�B���������ݲ�����CΪ�������Һ��

����˵���������
A. B��Ϊԭ��ص�����
B. A��B��C���ֱܷ�ΪZn��Cu��ϡ����
C. C����������A���ƶ�
D. A������������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ե�������һ����Ȼ�������������н�ǿ�Ŀ��������ԣ�����Ч���Ͱ�֢�Ͷ���Ӳ���ķ��ա���ṹ��ʽΪ��
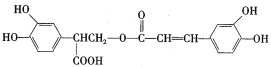
�ϳ�·�����£�
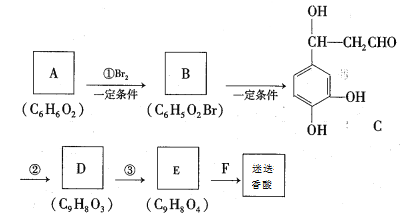
��������ش��������⣺
(1)A�Ľṹ��ʽΪ____________��
(2)�����ڵķ�Ӧ���ͷֱ���_____________��_____________��
(3)E�Ľṹ��ʽΪ__________________
(4)F��һ�������·������۷�Ӧ�Ļ�ѧ����ʽΪ__________________________________
(5)д��һ����C��Ϊͬ���칹�壬��ͬʱ���������������л��Ľṹ��ʽ________________
�ٱ����ϵ�һ�ȴ�����2��
��1 mol���л�������1 molNaHCO3��Ӧ
������Ũ��ˮ��Ӧ��1 mol���л�������3 mol Br2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£���6�����NO2(������N2O4)��һ�������NO�����Թ��У������Թܵ�����ˮ�У���ͨ��4.5�����O2��ַ�Ӧ��ʣ��1.5������壬��ԭNO�������Ϊ(����)
��3�������4�������5�������5.5�������2���
A.��B.��C.�ܻ��D.�ڻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪25��ʱ,������Һ�д���������ϵ:![]()
(1)�������Һ�м���һ��������ʱ,��ʽ�е���ֵ�Ƿ����仯_________ ?Ϊʲô_________?
(2)���������ʼŨ��Ϊ0.010 mol L-1,ƽ��ʱc(H+)�Ƕ���_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com