
����Ŀ��X��Y��T��Q��Z ����Ԫ�أ�λ��Ԫ�����ڱ�ǰ�����ڣ�Ԫ�ص����ʻ�ṹ��Ϣ�����
Ԫ�� | ���ʻ�ṹ��Ϣ |
X | ����Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ������µ������������ȶ�������ԭ�ӽϻ��� |
Y | ��̬ԭ�ӵ�3p�������4������ |
T | ԭ�Ӻ���s���������������p������������������ں�������Ԫ�أ����䵥���dz�������ȼ�� |
Q | ������������Ԫ����ԭ�Ӱ뾶��С |
Z | ��̬ԭ�ӵ�2��������M����ȫ���������� |
�����������Ϣ�ش��������⣺
��1��д��X3����һ�ֵȵ�����Ļ�ѧʽ___��
��2��д��TԪ�ػ�̬ԭ�ӵĺ�������Ų�ͼ___��
��3��Ԫ��X��T�ĵ縺����ȣ�___��С����Ԫ�����ƣ���Ԫ��X�ĵ�һ��������T��Ƚϣ�T��___�����С������
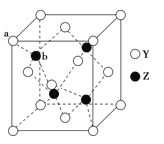
��4����ZԪ�������ڱ���λ��__����Z���ʾ�����Zԭ������ά�ռ���Ķѻ���ʽΪ___�ѻ���
��Z���Ȼ����백ˮ��Ӧ���γ������[Z��NH3��4��H2O��2]Cl2������������ʱ������ʧȥ�����е�������___��д��ѧʽ����
��5��Ԫ��X��Q���γɻ�����XQ3�����ݼ۲���ӶԻ��������ж�XQ3�Ŀռ乹��Ϊ___��������Xԭ�ӵ��ӻ���ʽΪ___�ӻ���
��6��Y��Z���γɻ����ᄃ��ľ�����ͼ��ʾ���û�����Ļ�ѧʽΪ___��
���𰸡�CO2 ![]() �� С ds �������ܶѻ� H2O ������ sp3 ZnS
�� С ds �������ܶѻ� H2O ������ sp3 ZnS
��������
X�γɵĵ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ������µ������������ȶ�������ԭ�ӽϻ��ã���XΪNԪ�أ���̬ԭ�ӵ�3p�������4�����ӣ������Ų�ʽΪ1s22s22p63s23p4,YΪSԪ�أ�Tԭ�Ӻ���s���������������p������������������ں�������Ԫ�أ����䵥���dz�������ȼ����ΪOԪ�أ�QΪ������������Ԫ����ԭ�Ӱ뾶��СΪClԪ�أ�Z��̬ԭ�ӵ�2��������M����ȫ���������ӣ�ΪZnԪ�أ��ݴ˻ش����⣻
�ɷ�����֪XΪNԪ����YΪSԪ�أ�TΪOԪ����QΪClԪ�أ�ZΪZnԪ����
��1��X3��ΪN3������N3����ԭ�Ӹ�����ͬ���۵�������ͬ��һ�ֵȵ�����ΪCO2��
��2��TΪOԪ�أ���̬��ԭ�ӵ�����������ԳɶԵ��ӣ�2���ɵ����ӣ���������Ų�ͼΪ![]() ��
��
��3��XΪNԪ�أ�TΪOԪ����ͬ���ڴ������ҵ縺������ǿ����һ�����������ʵ��ĵ縺�Խ�С�����ڵ�ԭ�������p���Ϊ������ṹ����һ�����ܽ���ԭ�Ӵ��ĵ�һ�����ܽϴ�
��4����ZΪZnԪ�أ������ڱ���λ��ds��������ά�ռ���Ķѻ���ʽΪ�������ܶѻ���
����λԭ�ӵ縺��Խ���������ӵ�����Խǿ���������ӶԺ�����Ԫ����ϵ�������Խ�����γɵ���λ��Խ����������ʧȥ���縺��O>N���������ȣ�����ʧȥ�������е�������H2O��
��5��Ԫ�ص����ȿ��г�NCl3�����ݼ۲���ӶԻ������ۿ�֪���÷���������ԭ�Ӽ۲���ӶԸ�����4���Һ���һ���µ��Ӷԣ����Կռ乹��Ϊ�����Σ������е�ԭ�ӵ��ӻ���ʽΪs p3�ӻ���
��6��YΪSԪ�أ�ZΪZnԪ����S2-λ�ڶ�������ģ�Zn2+λ���ڲ�������λ�ڶ��㣬ͬΪ8�����������У��˷�֮һ���ڸþ�����λ�����ϣ�ͬΪ2�����������У�����֮һ���ڸþ�����λ���ڲ������ڸþ���������S2-����=![]() =4��Zn2+����=4��������Ϊ1:1�����Ի�ѧʽΪZnS��
=4��Zn2+����=4��������Ϊ1:1�����Ի�ѧʽΪZnS��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25��ʱ����Ũ��Ϊ0.1000 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.1000 mol��L��1��������HX��HY��HZ�ζ�������ͼ��ʾ������˵����ȷ����
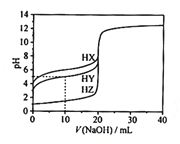
A.����ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX
B.���ݵζ����ߣ��ɵ�Ka(HY)��10��6
C.������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��c(X��)��c(Y��)��c(OH��)��c(Na+)��c(H��)
D.HY��HZ��ϣ��ﵽƽ��ʱ��c(H��)��c(Y��)��c(Z��)��c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ײʱ����ȫ�����з�����Ӧ��10NaN3��2KNO3=K2O��5Na2O��16N2��������˵������ȷ����( )
A.��ԭ�ԣ�NaN3 > N2
B.����65 g NaN3�μӷ�Ӧ�������ɵ�N2�����ʵ���Ϊ1.6 mol
C.ÿת��1 mol���ӣ������ɱ�״����N2�����Ϊ35.84 L
D.����ԭ��N�뱻������NΪ15 ��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ֲ���纣���������к��зḻ�ĵ�Ԫ�ء���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�

(1)ָ���Ӻ�������ȡI2��ʵ��������ƣ�
��____________����__________�ڵ����ӷ���ʽ__________����
(2)��ȡ��Ĺ����У��ɹ�ѡ����л��ܼ�����____����
A �ױ����ƾ� B ���Ȼ�̼����
C ���͡����� D ���͡�����
(3)Ϊʹ������I��ת��Ϊ����л���Һ��ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������ȱ�ٵ�������________��
(4)�Ӻ�����л��ܼ�����ȡ�⣬��Ҫ��������ָ����������װ���еĴ���֮��__________��
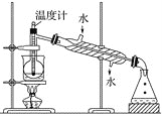
(5)�����������ʱ��ʹ��ˮԡ���ȵ�ԭ����_________________�����̬����________�С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���Ȼ�菉���ľ���(�����е���С�ظ���Ԫ)����֪���������������Cs�����Ӻ˼����Ϊa cm���Ȼ�蘆���Է�������ΪM��NAΪ�����ӵ����������Ȼ�菉����ܶ���(����)

A. ![]() g/cm3 B.
g/cm3 B. ![]() g/cm3 C.
g/cm3 C. ![]() g/cm3 D.
g/cm3 D. ![]() g/cm3
g/cm3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����װ���͡(I)���������Ƽ���Ѫ������ϳ��о�������Ҫ���壬�ϳ�·����ͼ��ʾ��
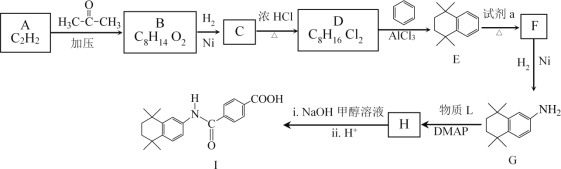
��֪��i.HC![]() CH+
CH+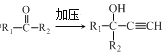
ii.R��NO2![]() R��NH2
R��NH2
iii.R1��NH2+![]() +HCl
+HCl
(1)A�����������_________��
(2)B�Ľṹ��ʽ��________��
(3)D��E�Ļ�ѧ����ʽ��__________��
(4)�Լ�a��_________��
(5)��֪H�ںϳ�I��ͬʱ�������ɼ״���G��H��������L�Ľṹ��ʽ��______��
(6)B��һ��ͬ���칹�����������������ṹ��ʽ��________��
���ܷ���������Ӧ
�ں˴Ź�������ֻ���������շ�
(7)D��E�Ĺ������ж��ָ�����������ڸ߷��ӻ�����Ľṹ��ʽ��_______��
(8)![]() Ҳ�Ǻϳ����װ���͡(I)��һ��ԭ�ϣ��ϳ�·����ͼ��ʾ����������������Ϣ���м����Ľṹ��ʽ��_______________��
Ҳ�Ǻϳ����װ���͡(I)��һ��ԭ�ϣ��ϳ�·����ͼ��ʾ����������������Ϣ���м����Ľṹ��ʽ��_______________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��A��B��F�Ǽ�ͥ�г������л��F������ʳƷ��װ��E��ʯ�ͻ�����չˮƽ�ı�־����������ת����ϵ�ش����⡣

��1���ֱ�д��A��E�й����ŵ����ƣ�A��_________��E��_________��
��2��������������Ϊ________________��
��3��д����Ӧ���ͣ���_________��
��4����д�����з�Ӧ�Ļ�ѧ����ʽ��
��д��A��B��Ũ�����м��ȷ�Ӧ�ķ���ʽ_________________��
��B�ڽ���ͭ�������ڿ����м��ȷ�Ӧ________________��
��5��F��һ�ֳ����ĸ߷��Ӳ��ϣ��������Ǵ����˾�ķ��㡣Ȼ�������ֲ�����ɵĵ����ijһ����������__________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʾ��Һ��Ũ�ȵķ���ͨ�������֣���Һ�����ʵ���������(w)�����ʵ���Ũ��(c)�������������Һʱ�����ݲ�ͬ����Ҫ���в�ͬ�����Ʒ������������ա�
(1)��10%(�ܶ�Ϊ1.01 g��cm��3)������������Һ���Ƴ�27.5 g 2% ������������Һ��
�ټ��㣺��________g 10%(�ܶ�Ϊ1.01 g��cm3)������������Һ�������Ϊ________mL�����________mLˮ(��ˮ��1 g��cm��3)����ϡ�͡�
����ȡ����________mL��Ͳȡ10% �������ƣ���ȡʱ����Ҫ����Ͳ________����ˮƽ��Ȼ�����ձ����________mL��Ͳ��ȡ����ˮҲע���ձ��
���ܽ⣺��________��������Һ������ȣ�����27.5 g 2% ������������Һ��
(2)��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ����ϡ�ͳ�3 mol��L��1��ϡ����100 mL���ش��������⣺
����ҪȡŨ����________mL��
�����Ʋ����ɷֽ�����¼�����������ȷ�IJ���˳����__________________________(����ĸ����ͬ)��
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C��������ȴ��ϡ����ע���Ѽ�鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹ��Һ����ǡ����̶�������
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���
(3)ʵ����������1 mol��L��1������������Һ��1 mol��L��1��������Һ��100mL��
��Ҫ��������������Һ������������ƽ��ȡ�������ƹ���ʱ����ƽ����Ϊ________��
A��4.0 g����������B��4.00 g�������� C����4.0 g
������������������Һ��������Һ�ĸ��������У������Բ�ͬ����__________��
A����������ȡ������B���ܽ��ϡ�� ��C����Һ��ϴ�ӡ���D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ̽��������������ʣ�����ͼ��ʾװ�ý���ʵ�顣

��ش��������⣺
��1��װ��A��ʢ���������Ƶ�����������__________����Ӧ�Ļ�ѧ����ʽΪ_______________��
��2��װ��B�е�������__________________����Ӧ�����ӷ���ʽΪ_________________��
��3��װ��C�е�������____________________��������˵������������е�������________________________��
��4��װ��D��Ŀ����̽������������Ʒ�����õĿ����ԣ�д��ʵ�����������_____________��
��5��β���ɲ���__________��Һ���ա�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com