
����Ŀ�������£�����Ũ�ȵ����������HA��HB��NaHCO3��Ӧ�ų�CO2�������ʱ��Ĺ�ϵ��ͼ��ʾ��������˵��������ǣ�( )

A. ���ԣ�HA<HB
B. pH��Ϊ4��HA��HB��Һ�к͵���NaOHʱ������HA��Һ���С
C. pH��Ϊ9��NaA��Һ��NaB��Һ��ȣ�NaA��Һ��ˮ�ĵ���̶ȴ�
D. Ũ�Ⱦ�Ϊ0.1mol/L��NaA��Һ��NaB��Һ��ȣ�NaA��Һ��ˮ�ĵ���̶ȴ�
���𰸡�C
��������
A.��ͼ���Կ�������Ũ�ȵ����������HA��HB��NaHCO3��Ӧ���ڿ�ʼһ��ʱ������ͬʱ��ų�CO2�����HB��HA�࣬˵��HA��Һ�е�c(H+)��HB��Һ�е�c(H+)С������HA<HB����A��ȷ��
B.��Ϊ����HA<HB������pH��Ϊ4��HA��HB��Һ��c(HA)![]() c(HB),�����к͵���NaOHʱ������HA��Һ���С����B��ȷ��
c(HB),�����к͵���NaOHʱ������HA��Һ���С����B��ȷ��
C.NaA��NaB��Ϊǿ�������Σ���Һ�Լ��ԣ�����pH��Ϊ9������ˮ�ĵ������ͬ����C����
D.Ũ�Ⱦ�Ϊ0.1mol/L��NaA��Һ��NaB��Һ��ȣ���������HA<HB������NaA��ˮ��̶ȴ�ˮ�ĵ���̶ȴ�D��ȷ��
�����ΪC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС��������װ����ȡ�ռ����������������о������ʡ���ش��������⡣

(1)װ�ü�������A��������_____________������Ӧװ����Һ��________________����װ���з�����Ӧ�����ӷ���ʽΪ__________________________________________________����ȡ�ռ�����������װ�ýӿ�����˳����a�� _____________��g(��������)��
(2)�Ʊ���Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ��Ӧ����Һ�������Ũ�ȣ�̽��С��ͬѧ�������ʵ�鷽����
I������������AgNO3��Һ��Ӧ���������ɵ�AgCl������
������������к͵ζ����ⶨ��
��������֪��CaCO3(����)��Ӧ������ʣ���CaCO3������
�̶����������жϺ�ʵ�飺
���ж�I���������У�������____________��
�ڽ��Т�ʵ�飺ȷ��ȡ������Һϡ��һ����������Ϊ������
a.��ȡ����20.00mL����ƿ�У�����ƿ�д�������ˮ����ʵ�����Ƿ���Ӱ�죿_____������������������)��0.10 molL-1NaOH����Һ�ζ�������NaOH����Һ�������ͼ��ʾ�������Ϊ_____ mL��

b��ƽ�еζ�����ʵ������
���жϢ���ʵ����___________(����ƫ��������ƫС������ȷ��)��[��֪��Ksp(CaCO3)��2.8��10-9��Ksp(MnCO3)��2.3��10-11
(3)ijͬѧ��Ϊ���������������Ե�ȱ�ݣ���ָ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ܱ������У���ӦaA(��)![]() bB(��)�ﵽƽ������¶Ȳ��䣬�������������һ�������ﵽ��ƽ��ʱ��B��Ũ����ԭ����60%����
bB(��)�ﵽƽ������¶Ȳ��䣬�������������һ�������ﵽ��ƽ��ʱ��B��Ũ����ԭ����60%����
A. ƽ�����淴Ӧ�����ƶ��� B. ����A��ת���ʼ�С��
C. ����B���������������� D. a>b
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���˴Ź����ױ�����³���� J(C13H20N2O2)�б�����ֻ�������⣬���ĺϳ�·�����£�

��ش��������⣺
(1)���� C ��������________________��F��G ��Ӧ������___________________��
(2)������Ҫ���������______________________________________________��
(3)��֪��³�����������(���ţ�BH��)�ĵ��볣�� Ka= 1��10-9��������� pH=7 ʱ���������տ�����Һ��� B �� BH����Ũ�ȱ�________��
(4)д�� D��E �Ļ�ѧ����ʽ________________________________________________��
(5)K �DZ� D ��һ��̼ԭ�ӵ�ͬϵ�д���������������� K ������ͬ���칹��Ľṹ��ʽ___________��
�����ڷ����廯����
������̼��������Һ��Ӧ
��H-NMR ����ʾ�� 5 ����ԭ��
(6)���������ϳ�·�ߺ���Ϣ�������� C�����ױ���HOCH2CH2N(C2H5)2 Ϊԭ��(�������Լ���ѡ)�ϳ�������³����(��֪�����ױ���ˮ���γɹ��л����)_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��25��ʱ��AgCl��KspΪ1.8��10��10��Ag2S��KspΪ6.3��10��50����AgCl��Ag2S�ı�����Һ�������ϣ��ټ�������ŨAgNO3��Һ�������ķ�ӦΪ( )
A. ֻ��Ag2S�������� B. AgCl��Ag2S�����ʵ�������
C. AgCl��������Ag2S���� D. Ag2S��������AgCl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(ClNO)���л���ϳ��е���Ҫ�Լ�������NO��Cl2�ڳ��³�ѹ�·�Ӧ�õ���ClNO�����������£���ɫ���壬�۵㣺��59.6�棬�е㣺��6.4�棬��ˮ��ˮ�⡣��֪��HNO2�������������л�ԭ�ԣ�AgNO2����ˮ�����������AgNO2+HNO3= AgNO3+ HNO2��
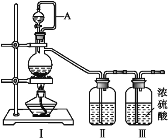
(1)���â�װ���Ʊ�ԭ����NO��Cl2
�� д������װ�â��Ʊ����������ӷ�Ӧ����ʽ��_________��
�� ��������װ���Ʊ�NOʱ������ʢװ����Ϊ_________(д��ѧʽ)��
(2)��������װ���ڳ��³�ѹ���Ʊ�ClNO
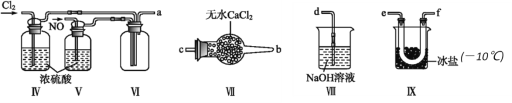
�� װ������˳��Ϊa��________(�������������ҷ�����Сд��ĸ��ʾ)��
�� ΪʹNO��Cl2ǡ����ȫ��Ӧ����ClNO��������ͨ��NO��Cl2������������ٱ�Ϊ_________��
�� װ�â���������_____________��
�� װ�â�����β��ʱ����ͬѧ��Ϊβ���е�ij�����岻����ȫ�����գ�Ϊ�˳������β�����ɽ�β����______________(����)ͬʱͨ��NaOH��Һ�С�
�� ��ˮ��Ũ�����Ũ����Ļ��ᣬһ�������»��������ClNO��Cl2���÷�Ӧ�Ļ�ѧ����ʽΪ______________________________________��
�� д����֤ClNO��H2O��ȫ��Ӧ�����Һ�д���Cl-��HNO2��ʵ�鲽�裺ȡ������Ӧ�����Һ���Թ��У�______________________________________________________��(��ѡ�Լ����£�AgNO3��Һ��ϡ���ᣬKMnO4��Һ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������H�ĺϳ�·�����£�
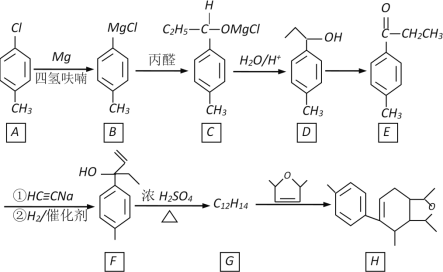
��֪CH��CH��NaNH2Һ�������¿�����CH��CNa��NaC��CNa
(1)A�Ļ�ѧ������________________________��B��C�ķ�Ӧ������________________
(2)D��E�ķ�Ӧ�Լ���������________��F��������������������____________
(3)H�ķ���ʽ��___________________
(4)F��G�Ļ�ѧ����ʽ��________________
(5)W��E��ͬϵ���E��һ��̼ԭ�ӣ����������������W��ͬ���칹��Ľṹ��ʽ��___________(дһ��)
�������ֹ�����
����FeCl3��Һ����ɫ
�ۺ˴Ź�������������壬�����֮����3�U2�U2�U2�U1
(6)����������Ŀ��Ϣ��д������ȩ����ȲΪԭ�ϣ��Ʊ�������![]() �ĺϳ�·��(���Լ���ѡ)_________��
�ĺϳ�·��(���Լ���ѡ)_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijһԪ���ᣨ��HA��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�HA![]() H++A-���ش��������⣺
H++A-���ش��������⣺
��1������Һ�м�������NaA���壬����ƽ�⽫��______________������������桱����Ӧ�����ƶ���
��2��������Һ�м�������NaCl��Һ������ƽ�⽫��______________����������������������������ƶ������ƶ�����ҺpH��______________�������������С�����䡱������Һ��![]() ��ֵ______________�����������С�����䡱����
��ֵ______________�����������С�����䡱����
��3����25���£���a molL-1�İ�ˮ��0.01molL-1������������ϣ���Ӧƽ��ʱ��Һ��c��NH4+��=c��Cl-��������Һ���������ú�a�Ĵ���ʽ��ʾNH3H2O�ĵ��볣��Kb=______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ���ճ������������������Ҫ��Ӧ�á�����˵������ȷ����(����)
A. ij��ˮ��Ʒ�ɼ������һ��ʱ�䣬pH��4.68��Ϊ4.28������Ϊ��Һ�е�SO32-ˮ��
B. ����ˮ���γɵ�Al(OH)3����������ˮ�������������ˮ�ľ���
C. ������FeCl3��Һ�����ˮ�п��Ʊ�Fe(OH)3���壬���õ�������ˮ��ԭ��
D. ����FeCl3��Һʱ�������������ᣬ����Fe3��ˮ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com