
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ,�á�84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش���������:
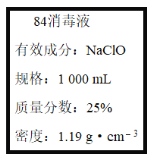
��1��ijͬѧȡ100 mL�á�84����Һ��,ϡ�ͺ���������,ϡ�ͺ����Һ��c(Na+)��____mol��L-1��
��2����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ������˵����ȷ����____(����ĸ)��
a.��ͼ��ʾ�������У��������Dz���Ҫ�ģ�����Ҫһ�ֲ�������
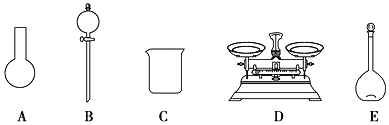
b.����ƿ������ˮϴ����Ӧ��ɺ����������Һ����
c.���ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
d.��Ҫ����NaClO���������Ϊ143.0 g
���𰸡� 0.04 c
����������1������c=1000�Ѧ�/M������Һ�����ʵ���Ũ�ȣ�����ϡ���������ʵ���������㣻
��2��a����������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ������
b������ƿ���ܺ�ɣ�
c�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС��
d������m��cVM������Ҫ���ʵ�������
��1���á�84����Һ�������ʵ���Ũ��c��1000��1.19��25%/74.5 mol��L��1��4.0mol/L��ϡ���������ʵ����ʵ������䣬��ϡ�ͺ�������Ƶ����ʵ���Ũ����4.0mol/L��100��0.04mol/L������ϡ�ͺ����Һ��c(Na+)��0.04 mol��L-1��
��2��a������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��������ȡ�����ܽ⣨ϡ�ͣ�����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������������ƽ����Ͳ����ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����Ҫ���ǣ�Բ����ƿ�ͷ�Һ©��������Ҫ����������ͷ�ιܣ�a����
b������ƿ������ˮϴ������������Һ���ƣ�����ƿ���ܺ�ɣ�b����
c�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС������c��n/V��֪��ҺŨ��ƫ�ͣ�c��ȷ��
d������480mL��NaClO��������Ϊ25%������Һ�������õ�����ƿ�������ʵ���Ũ��Ϊ4.0mol/L����Ҫѡ��500mL����ƿ��������Ҫ���ʵ�����m=4.0mol/L��74.5g/mol��0.5L=149g��d����
��ѡc��


 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ǿ������Һ���ܴ����������ɫ���������ǣ� ��
A.K����Na����NO3����MnO4��B.Mg2����Na����Cl����SO42��
C.K����Na����Br����Cu2��D.Na����Ba2����OH����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��800��ʱ��2L�ܱ������ڷ�Ӧ��2NO��g��+O2��g��![]() 2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��1����ƽ��ʱNO��ת����_________________��
��2����ͼ�б�ʾNO2�ı仯��������________����O2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v��O2��=____________��

��3����˵���÷�Ӧһ���ﵽƽ��״̬����_____________��
a��v��NO2��=2v��O2�� b����������ɫ���ֲ���
c��2v����NO��=v����O2�� d��������ѹǿ���ֲ���
��4��������÷�Ӧ�ķ�Ӧ���ʵ���___________________��
a����ʱ�����NO2���� b���ʵ������¶�
c������O2��Ũ�� d��ѡ���Ч����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������״���෨������9�����ʽ��з��࣬Ȼ��Ҫ��ش����⣺
(1)���з���(�����������������Ӧ������)��������Ӧ�ո�����_________��
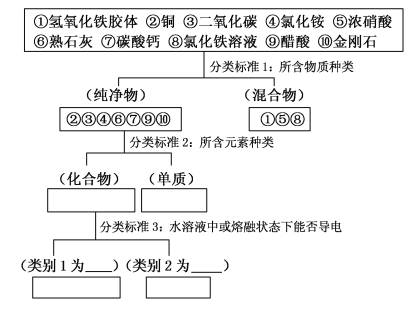
(2)��������������Ȼ�����Һ������Ϻ��ѽ������֣�����Ϊ��ͨ��ʲôʵ�������֣�__________(��Ҫ˵��ʵ�鷽�����ж�����)��
(3)д������10�������е�ǿ��ǿ���ϣ������кͷ�Ӧ�Ļ�ѧ����ʽ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����CuSO4��Һ�м���H2O2��Һ���ܿ��д��������ݳ���ͬʱ���ȣ�һ��ʱ�����ɫ��Һ��Ϊ��ɫ����(Cu2O)����������H2O2��Һ����ɫ�����ֱ�Ϊ��ɫ��Һ�������Ӧ���Է�����Ρ����й����������̵�˵������ȷ����
A. Cu2+��H2O2�ֽⷴӦ�Ĵ��� B. H2O2ֻ������������
C. H2O2�ĵ���ʽΪ: ![]() D. �����˷�ӦCu2O + H2O2+4H+=2Cu2++3H2O
D. �����˷�ӦCu2O + H2O2+4H+=2Cu2++3H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�ǽ���������(S)�ǵ���ɫ�����ĩ,������ˮ��Ϊ����֤��Ԫ�صķǽ����Ա���Ԫ�صķǽ�����ǿ����ʾ��Na2S +2HCl = 2NaCl+ H2S����,ij��ѧʵ��С�����������ʵ��,��ش���������:
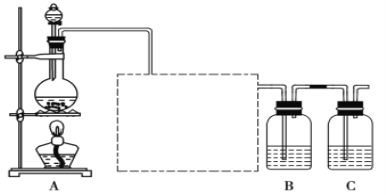
(1)�������߿��ڵ�ʵ��װ��ͼ____,�����Լ�Ϊ___________,��װ�õ�������__________��
(2)װ��B��ʢ�ŵ��Լ���____(�����)��
A.Na2S��ҺB.Na2SO3��ҺC.Na2SO4��Һ
Bװ���з�����Ӧ�����ӷ���ʽΪ___________________________��
(3)��֪:����ԭ������ԭ�ӵĵ��Ӳ�����ͬ,�ȵ�ԭ�Ӱ뾶С����ԭ�ӡ�������������Ա�����ǿ;�����������ڼ��������·�Ӧ�������Ȼ���,�������ڼ��������·�Ӧ����������;��HCl��H2S�ȶ�;������ȴ������ȶ�;��˵���ȵķǽ����Ա���ǿ����____(�����)��
A.ȫ�� B. �٢ڢۢ� C.�٢ڢܢ� D. �٢ۢܢ�
(4)װ��C��ʢ���ռ���Һ,Ŀ�������շ�Ӧ��ʣ�������,��ֹ��Ⱦ����,д����װ������������Ӧ�����ӷ���ʽ:_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣���ش��й�������
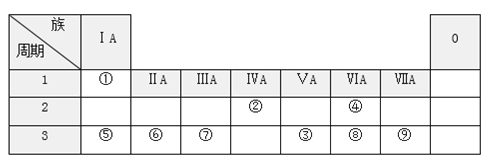
�밴Ҫ��ش��������⣺
(1)Ԫ�آߵ�ԭ�ӽṹʾ��ͼ______________________���ɢڡ�������Ԫ����ɵĻ�������___________������(��������������������)
(2)Ԫ�آ١��ܡ�������֮������γ��������͵Ļ����д��һ�ֹ��ۻ�����Ļ�ѧʽ__________________ ��һ�����ӻ�����Ļ�ѧʽ_________
(3)�ܢݢ�����Ԫ�ص����Ӱ뾶�ɴ�С��˳����____________(�����ӷ���)��
(4)��͢�����Ԫ�ص�����������Ӧ��ˮ���������Խ�������________(�ѧʽ)��
(5)Ԫ�آ۵���̬�⻯���Ԫ�آ����̬�⻯����ȶ�����___________(�ѧʽ)��
(6)Ԫ�آݵ�����������Ӧ��ˮ������Ԫ�آߵ�����������Ӧˮ���ﷴӦ�������ӷ���ʽΪ_________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��������A��B�ͼס��ҡ������ֻ���������ͼ��ʾ��ת����ϵ(���ֲ���δ�г�)������A����ΪʳƷ��װ���ϣ��������������
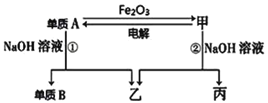
����ͼʾת����ϵ�ش�
(1)д������A��Fe2O3��Ӧ�Ļ�ѧ����ʽ__________________________________
(2)д�������Ƶ���A�Ļ�ѧ����ʽ__________________________________
(3)ʵ������ɵ���A��Fe2O3��Ӧʵ�飬��Ҫ���Լ�����_______��
a��KCl���������� b��KClO 3������ c��MnO2������ d��Mg
(4)���õ���A��Fe2O3��Ӧ��ԭ������ҵ�Ͽ�����___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ����(N2H4��H2O)����ɫ����ǿ��ԭ�Ե�Һ�壬ʵ�����Ʊ�ˮ���µ�ԭ��Ϊ��CO(NH2)2+2NaOH+NaClO=Na2CO3+N2H4��H2O+NaCl�ݴˣ�ijѧ�����������ʵ�顣
���Ʊ�NaClO��Һ��ʵ��װ������ͼͼ����ʾ�����ּг�װ����ʡ�ԣ�
��֪��3NaClO![]() 2NaCl+NaClO3
2NaCl+NaClO3
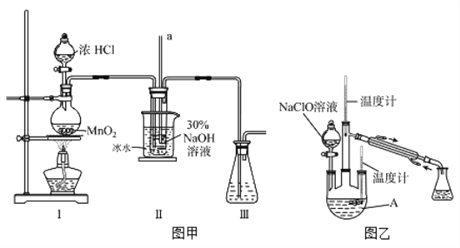
��1������30 %NaOH��Һʱ�����貣����������Ͳ���__________������ĸ����
A.����ƿ B���ձ� C����Һ�� D.������
��2��װ��I�з��������ӷ�Ӧ����ʽ��_______________�����в�����a������Ϊ____________�������ñ�ˮԡ�����¶���30�����£�����ҪĿ��___________________
����ȡˮ���¡�ʵ��װ������ͼͼ����ʾ
��3����Ӧ�����У������Һ©������Һ�ĵ��ٹ��죬 ����N2H4��H2O����A�з�Ӧ������������������Ʒ������˽��ͣ���д�����Ͳ��ʵ���ػ�ѧ��Ӧ����ʽ____________________����ַ�Ӧ��������A�ڵ���Һ���ɵõ�ˮ���µĴֲ�Ʒ��
���ⶨ�µĺ�����
��4����ȡ���0.3000 g����ˮ���20.0 mL��Һ��һ����������0.1500 mol��L-1��I2��Һ�ζ�����֪: N2H4��H2O + 2I2 = N2��+ 4HI + H2O��
�ٵζ�ʱ������ѡ�õ�ָʾ��Ϊ____________��
��ʵ��������I2��Һ��ƽ��ֵΪ20. 00 mL�������N2H4��H2O����������Ϊ____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com