
����Ŀ������Ŀ��������������ȷ���� ( )
A. ����������ˮ��ȥ��Ч����ǿ��˵�������ˮ�ⷴӦ�����ȷ�Ӧ
B. NH3(g) + HCl(g) = NH4Cl(s) �ڽϵ��¶������Է����У�˵���÷�Ӧ�Ħ�H>0
C. 500�桢30 MPa�£���7 g N2��3 g H2�����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪN2(g)��3H2(g)![]() 2NH3(g) ��H����38.6 kJ��mol��1
2NH3(g) ��H����38.6 kJ��mol��1
D. �����ȼ���ȣ���H��Ϊ��890.3 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4(g)��2O2(g)�� CO2(g)��2H2O(g)����H����890.3 kJ��mol��1
���𰸡�A
��������A. ����������ˮ��ȥ��Ч����ǿ��˵�������¶ȴٽ���ˮ�⣬ʹˮ��ƽ�������ƶ������Կ���˵�������ˮ�������ȷ�Ӧ����A��ȷ��B. ���ݡ�G����H��T��S��֪������GС��0ʱ��Ӧ�Է����У����ڷ�ӦNH3(g) + HCl(g) = NH4Cl(s)����ֵ��С�ķ�Ӧ����������÷�Ӧ�ڽϵ��¶������Է����У���˵���÷�Ӧ�ġ�H��0����B����C.��ϳɰ��ķ�Ӧ�ǿ��淴Ӧ��������ȷ����7 g N2��3 g H2�����ܱ������г�ַ�Ӧ���������˶���NH3(g)����������÷�Ӧ���ʱ䣬��C����D. ȼ����ָ����1mol��������ȫȼ�������ȶ���������ʱ���ų����������������ɵ�ˮӦΪҺ̬��������ȼ�յ��Ȼ�ѧ����ʽӦ��ʾΪCH4(g)��2O2(g)�� CO2(g)��2H2O(l)����H����890.3 kJ��mol��1����D����ѡA��


 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽��������

����������ȡ30mL0.50 molL-1���ᵹ��С�ձ��У���������¶���
������һ��Ͳ��ȡ50mL0.50molL-1NaOH��Һ������ͬһ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
��1�������30mL0.50mol/L������70mL0.50mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������___________�������������������������������к���___________����������������������������������_____________________��
��2��ijʵ��С������0.50mol/LNaOH��Һ����ʵ���д�ԼҪʹ��245mLNaOH��Һ��������Ҫ����NaOH����_____________________g��
��3������KOH����NaOH���Բⶨ���___________(����������������)Ӱ�������ô������HCl��ʵ�飬�Բⶨ���_____________(����������������)Ӱ����
��4����������Ϊ0.50 molL-1�����0.50 molL-1NaOH��Һ�ܶȶ���1g��cm3���кͺ���Һ�¶�������4����������Һ�ı�����c=4.18J��(g����)-1�����к�����H=______��С�������һλ��Ч��������
������ʵ������-57.3kJ��mol-1��ƫ�����ƫ���ԭ�������____________������ĸ����
A.ʵ��װ�ñ��¸���Ч����
B.��ȡNaOH��Һ���ʱ���Ӷ���
C.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
D.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳⶨ������¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F ��ԭ��������������Ķ���������Ԫ�ء�E ��ͬ���ڽ�������ǿ��Ԫ�ء������ҡ�����������������������Ԫ���е����ֻ�����ɵĻ��������֮���ת����ϵ��ͼ��ʾ�����м��������еĵ�ζƷ�����ǵ���ɫ���塣����˵������ȷ����

A. Ԫ��B���⻯����ܾ�����������ṹ
B. Ԫ���ϵ��������Ӧˮ�ﻯ������һ�������������ǿ
C. ԭ�Ӱ뾶:r(E)>r(F)>r(C)>r(D)
D. ������A4BC2D�ȿ����ǹ��ۻ����Ҳ�������ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ������ȷ���� �� ��
A. NaHCO3��ˮ�⣺HCO3����H2O![]() H3O����CO32��
H3O����CO32��
B. CaCO3�ĵ��룺CaCO3![]() Ca2+��CO32-
Ca2+��CO32-
C. ̼��Ƶ��ܽ�ƽ�⣺CaCO3(s)![]() Ca2��(aq)��CO32��(aq)
Ca2��(aq)��CO32��(aq)
D. Na2S��Һ��ˮ�⣺S2-��2H2O![]() H2S��2OH-
H2S��2OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȫ��������ԭ�����һ�����Ϳɳ��أ���ͬ��̬�ĺ���������Ϊ���������Ļ������ʣ��ֱ��ڸ��Ե����Ե��Һ�����С���ṹԭ����ͼ��ʾ���õ�طŵ�ʱ���Ҳ��еĵ缫��ӦΪ��V2+-e-=V3+������˵����ȷ����

A. �ŵ�ʱ���Ҳ۷�����ԭ��Ӧ
B. �ŵ�ʱ����۵ĵ缫��Ӧʽ��VO2++2H++e-=VO2++H2O
C. ���ʱ��ÿת��1mol���ӣ�n(H+)�ı仯��Ϊ1mol
D. ���ʱ���������ҺpH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ϩ�������˵���У�����ȷ����
A.��ϩ���ڲ����������������ڱ�����
B.��ϩ������ԭ�Ӵ���ͬһƽ�棬������ԭ�Ӳ���ͬһƽ��
C.��ϩ�����е�̼̼˫����������̼̼�������ȶ�����������ѧ��Ӧ
D.������ʹ���Ը��������Һ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л��������˵����ȷ����
A. ����ʽΪC3H6Cl2���л�����4��ͬ���칹��(�����������칹)
B. ![]() ��
��![]() ��Ϊͬϵ��
��Ϊͬϵ��
C. ��Ȳ��������Ȼ�̼��Һ��Ӧ����1,2-��������
D. �ױ�����������ԭ�Ӷ���ͬһƽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڵ�����������м������ʯ(�á�A������)��������Al2O3�۵�����á�����ʯ������ԭ��Ϊ2Al(OH)3��12HF��3Na2CO3=2A��3CO2����9H2O�������������������գ�
��1������ʯ�Ļ�ѧʽΪ____________�� �������Ӽ���____________�Ȼ�ѧ����
��2���������к���10�����ӵķ�����________(д����ʽ)���÷��ӵĿռ乹��_______������ԭ�ӵ��ӻ���ʽΪ___________________��
��3����Ӧ���е縺������Ԫ��Ϊ________(��Ԫ�ط���)��д����ԭ�������ĵ����Ų�ͼ��_______________��
��4������ʯ�����������ɣ�����ʯ�ľ����ṹ��ͼ����ʾ����λ�ڴ�������Ķ�������ģ���λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ�������������__________(��������)��
��5��Al���ʵľ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��
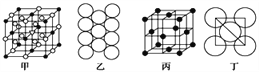
����֪Al��ԭ�Ӱ뾶Ϊd��NA���������ӵ�������Al�����ԭ������ΪM����һ��������Alԭ�ӵ���ĿΪ___________���� Al������ܶ�Ϊ________(����ĸ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��HCN(aq)��NaOH(aq)��Ӧ����H=��12.1kJ /mol��HCl(aq)��NaOH(aq)��Ӧ����H =��55.6kJ/ mol����HCN��ˮ��Һ�е������H����
A. ��67.7 kJ /mol B. ��43.5kJ /mol C. +43.5 kJ/ mol D. +67.7 kJ/ mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com