
����Ŀ��һ���¶��£���1.0L���ܱ������м���0.60molX��g����������ӦX(g)![]() Y(s)+2Z(g)����÷�Ӧ��X��Ũ���뷴Ӧʱ��Ĺ�ϵ�����ʾ��
Y(s)+2Z(g)����÷�Ӧ��X��Ũ���뷴Ӧʱ��Ĺ�ϵ�����ʾ��
��Ӧʱ��t/min | 0 | 1 | 2 | 3 | 4 | 6 | 8 |
c(X)/(mol��L-1) | 0.60 | 0.42 | 0.30 | 0.21 | 0.15 | a | 0.0375 |
(1)0��3min����Z��ʾ��ƽ����Ӧ�ٶ�v(Z)=___��
(2)�����÷�Ӧ�з�Ӧ���Ũ����ʱ��Ĺ�ϵ���ó��Ľ�����___���ɴ˹����Ƴ���6minʱ��Ӧ��X��Ũ��Ϊ___mol��L-1��
(3)�÷�Ӧ���淴Ӧ������ʱ��仯��������ͼ��ʾ��t2ʱ�ı������������___��___��

���𰸡�0.26mol��L-1��min-1 ÿ��2minX��Ũ�ȼ���Ϊԭ����һ�� 0.075 ����Z ������ϵ��ѹǿ
��������
(1)0��3min�ڿ������X��ʾ��ƽ����Ӧ���ʣ�Ȼ�����û�ѧ��������ϵ�����Z��ʾ��ƽ����Ӧ�ٶ�v(Z)��
(2)�����÷�Ӧ�з�Ӧ���Ũ����ʱ��Ĺ�ϵ��Ѱ�ҹ������ݵĹ����ԣ��ɴ˵ó��Ľ��ۡ��ɴ˹��ɿ��Ƴ���6minʱ��Ӧ��X��Ũ�ȡ�
(3)���ݷ�Ӧ��t2ʱ�ı��������Ũ�ȡ�ѹǿ���¶ȡ��������������з�����
(1)0~3min�ڣ�c(X)=(0.60-0.21)mol/L=0.39mol/L��ƽ����Ӧ����v(X)=![]() = 0.13mol��L-1��min-1����v(Z)=2v(X)����v(Z)=0.26mol��L-1��min-1����Ϊ��0.26mol��L-1��min-1��
= 0.13mol��L-1��min-1����v(Z)=2v(X)����v(Z)=0.26mol��L-1��min-1������0.26mol��L-1��min-1��
(2)���ݱ������ݿ�֪��ÿ��2min��X��Ũ�ȼ���Ϊԭ����һ�룬�ɴ˹����Ƴ���6minʱ��Ӧ��X��Ũ��Ϊ0.075 mol��L-1����Ϊ��0.075��
(3)![]() ʱ�̣�
ʱ�̣�![]() ˲�������ܵ�ԭ���Ǽ���������Z��������ϵ��ѹǿ����Ϊ������Z��������ϵ��ѹǿ��
˲�������ܵ�ԭ���Ǽ���������Z��������ϵ��ѹǿ����Ϊ������Z��������ϵ��ѹǿ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʹ������һ������������ķ����忹��ҩ����ϳ�·�����£�
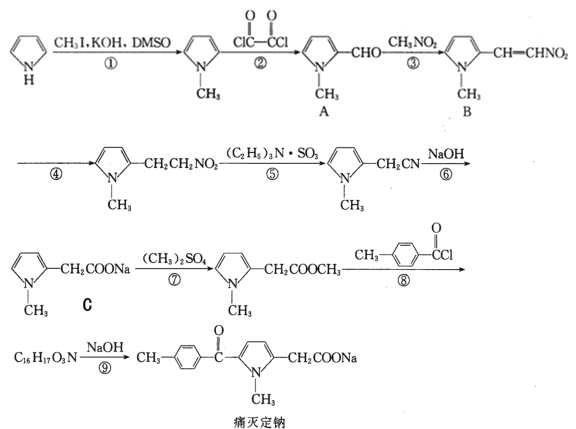
�ش��������⣺
(1)������ C �к��������ŵ�������_____��
(2)��ѧ��Ӧ�ٺܵ͢ķ�Ӧ���ͷֱ�Ϊ_____��_____��
(3)���й���ʹ���Ƶ�˵����ȷ����_____��
a��1mol ʹ�����������ӳ�������� 7molH2 b���˴Ź����������ܹ���ʾ 6 ���� c�����ܹ�������ԭ��Ӧ d�������ּӳɺ���������������� e����ֱ�ߵ�̼ԭ������� 4 ��
(4)��Ӧ��Ļ�ѧ����ʽΪ_____��
(5)�����廯���� X ����Է��������� A �� 14���� FeCl3 ��Һ����ɫ�Ľṹ����_____�֣������������칹�����˴Ź�����������ʾ�� 5 ����� X �Ľṹ��ʽ��_____��
(6) ���ݸ������ṩ�������Ϣ��д���ɻ�����![]() ����Ҫ���Լ��Ʊ��л�������
����Ҫ���Լ��Ʊ��л�������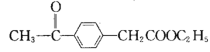 �ĺϳ�·��ͼ��________________
�ĺϳ�·��ͼ��________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽���÷�Ӧԭ������������ʵ�飺���ݻ�Ϊ1L���ܱ������У�����1mol CO2��3mol H2����500���·�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��ʵ����CO2��CH3OH(g)�����ʵ���(n)��ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g)��ʵ����CO2��CH3OH(g)�����ʵ���(n)��ʱ��仯��ͼ��ʾ��
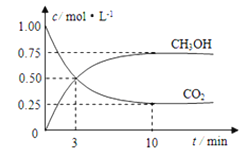
(1)�ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)=________________����ͼ�Ǹı��¶�ʱ��ѧ��Ӧ������ʱ��仯��ʾ��ͼ����÷�Ӧ������ӦΪ____________��Ӧ������ȡ������ȡ�����
(2)500��÷�Ӧ��ƽ�ⳣ��Ϊ______��������λС������������¶ȵ�800����У���ƽ��ʱ��Kֵ______�����������С�����䡱����
(3)500�������£����ijʱ�̣�CO2(g)��H2(g)��CH3OH(g)��H2O(g)��Ũ�Ⱦ�Ϊ0.5mol/L�����ʱv(��)______v(��)�����������������=������
(4)���д�ʩ��ʹ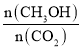 �������______��
�������______��
A�������¶� B����ԭ�����г���1molHe
C����ˮ��������ϵ�з���� D����С�����ݻ�������ѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���(Li)-������صĹ���ԭ����ͼ��ʾ������˵������ȷ����( )
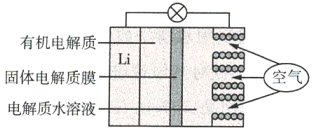
A.�����������������������Ӧ
B.Li+ͨ���л��������ˮ��Һ���ƶ�
C.�����ĵ缫��Ӧ��O2+4e-=2O2-
D.����ܷ�Ӧ��4Li+O2+2H2O=4LiOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ����
A.2.8g N2 �к��еĹ��õ��Ӷ���Ϊ 0.1NA
B.���³�ѹ�£�124��P4������P-P����ĿΪ6 NA
C.32������������������ȼ��ת�Ƶ�����Ϊ6 NA
D.�ڱ�״���£�22.4 L H2O �к�����ԭ����Ϊ NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
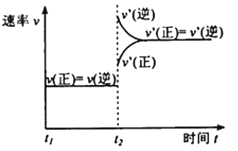
����Ŀ��ij�¶��£���һ���������н������·�Ӧ A(g)+3B(g) ![]() 2C(g)���������һ����˵����Ӧ�Ѵﵽƽ�����
2C(g)���������һ����˵����Ӧ�Ѵﵽƽ�����
�ٵ�λʱ���ڣ���1molB��Ӧ��ͬʱ��2molC����
��������ѹǿ����ʱ����仯
�۵�λʱ���ڣ���2molC���ɣ�ͬʱ��1molA����
���� A��B��C��ʾ�ĸ÷�Ӧ�Ļ�ѧ��Ӧ����֮��Ϊ 1��3��2
�������ƽ��Ħ����������ʱ����仯��������ܶȲ���ʱ����仯
A.�٢ܢ�B.�٢ڢ�C.�٢ڢ�D.�ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��SO2�ĺ����Ǻ��������Ⱦ��һ����Ҫָ�꣬��ҵ�ϳ����ô���ԭ�������շ�����SO2�����ô���ԭSO2����������SO2��Ⱦ�����ҿɵõ��о��ü�ֵ�ĵ���S��
��1���ڸ�����ִ��������£�CH4��ʹSO2ת��ΪS��ͬʱ����CO2��H2O��
��֪CH4��S��ȼ����(��H)�ֱ�Ϊ-890.3k/mol��-297.2kJ/mol����CH4��SO2��Ӧ���Ȼ�ѧ����ʽΪ_____________��
��2����H2��ԭSO2����S�ķ�Ӧ��������ɣ���ͼ1��ʾ���ù�����������ʵ����ʵ���Ũ����ʱ��ı仯��ϵ��ͼ2��ʾ:
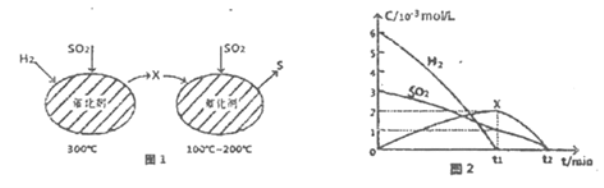
�ٷ�����֪XΪ______(д��ѧʽ)��0��t1ʱ��ε��¶�Ϊ_____��0��t1ʱ�����SOz��ʾ�Ļ�ѧ��Ӧ����Ϊ________��
���ܷ�Ӧ�Ļ�ѧ����ʽΪ_____________��
��3����̿����ԭSO2����S2,��ѧ����ʽΪ:2C(s)+2SO2(g)![]() S2(g)+2CO2(g)�����������У�1mol/LSO2�������Ľ�̿��Ӧ��SO2��ת�������¶ȵı仯��ͼ3��ʾ��
S2(g)+2CO2(g)�����������У�1mol/LSO2�������Ľ�̿��Ӧ��SO2��ת�������¶ȵı仯��ͼ3��ʾ��

�ٸ÷�Ӧ����H____0(����>������<��)
����a���ƽ�ⳣ��Ϊ_________��
��4����ҵ�Ͽ���Na2SO3��Һ���շ�����SO2,25��ʱ��1mo/L��Na2SO3��Һ����SO2������ҺpH=7ʱ����Һ�и�����Ũ�ȵĴ�С��ϵΪ________����֪:H2SO3�ĵ��볣��K1=1.3��10-2��K2=6.2��10-8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F���ֶ�����Ԫ�ص�ԭ��������������Ԫ��A��ij�ֺ��������ӣ�BԪ�ص��������������۴�����Ϊ2��CԪ�غ�EԪ��ͬ���壬��EԪ�ص���ۺ�����Ϊ��Ԫǿ�ᣬDԪ����ͬ����Ԫ�������Ӱ뾶��С��Ԫ�ء���ش��������⣺
��1��A��BԪ����ɵĻ�����B2A4�ĵ���ʽΪ _______________��
��2��D������������F����ۺ����ᷴӦ�����ӷ���ʽ________________________��
��3��CԪ�غ�EԪ�ص���ͼ��⻯��ķе�C __________E(����ڻ�С��)������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ھ����˵����ȷ������ǣ� ��
�ٷ��Ӿ����ж����ڹ��ۼ�
���ھ�����ֻҪ�������Ӿ�һ����������
�۽��ʯ��SiC��NaF��NaCl��H2O��H2S������۵����ν���
�����Ӿ�����ֻ�����Ӽ�û�й��ۼ������Ӿ����п϶�û�����Ӽ�
��CaTiO3������(�����ṹ��ͼ��ʾ)ÿ����ԭ�Ӻ�12����ԭ�ӽ�����

��SiO2������ÿ����ԭ����������ԭ���Թ��ۼ�����
�߾����з��Ӽ�������Խ����Խ�ȶ�
���Ȼ����ۻ�ʱ���Ӽ����ƻ�
A.�٢ڢۢ�B.�٢ڢ�C.�ۢݢ�D.�ۢݢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com