
����Ŀ������ΪԪ�����ڱ��е�һ���֣��û�ѧʽ��Ԫ�ط��Żش��������⡣
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� | �� |
��1��10��Ԫ���У���ѧ��������õ���__________��
��2���٢ڢ��У�����������ˮ���������ǿ����__________��
��3��10��Ԫ��������������ˮ���������ǿ����__________��
��4��Ԫ�آ���ɵĺ��Ǽ��Լ��ķ��ӵĵ���ʽ��__________��
��5�����֢١��ڵ�̼�����εļ�ʵ�鷽��__________��
��6���ٺ͢�����������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ__________��
��7���۵ĵ��������������ﷴӦ�ķ���ʽ��__________��
���𰸡�Ar KOH HClO4 ![]() ��ɫ��Ӧ Al(OH)3+OH-�TAlO2-+2H2O CO2+2Mg
��ɫ��Ӧ Al(OH)3+OH-�TAlO2-+2H2O CO2+2Mg![]() 2MgO+C
2MgO+C
��������
�����ڱ��и�Ԫ�ص����λ�ÿ�֪����ΪNaԪ�ء���ΪKԪ�ء���ΪMgԪ�ء���ΪCaԪ�ء���ΪAlԪ�ء���ΪCԪ�ء���ΪOԪ�ء���ΪClԪ�ء���ΪBrԪ�ء���ΪArԪ�ء�
��1��10��Ԫ���У���ѧ��������õ���ϡ������ArԪ�أ��ʴ�Ϊ��Ar��
��2��Ԫ�صĽ�����Խǿ������������ˮ����ļ���Խǿ��Na��K��Ca����Ԫ���У�KԪ�صĽ�������ǿ��������������ˮ����ļ�����ǿ����KOH���ʴ�Ϊ��KOH��
��3��10��Ԫ��������������ˮ�����������ǿ���Ǹ����ᣬ��ѧʽΪHClO4���ʴ�Ϊ��HClO4��
��4����ΪOԪ�أ�˫��ˮΪ���зǼ��ԵĹ��ۻ�������ӵĵ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��5��̼���Ƶ���ɫ��Ӧ��ɫ������ɫ�ܲ���Ƭ̼��ص���ɫ��ӦΪ��ɫ�����������ɫ��Ӧ����̼���ƺ�̼��أ��ʴ�Ϊ����ɫ��Ӧ��
��6����������Ϊ�����������������������ǿ����Һ��Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪAl(OH)3+OH-�TAlO2-+2H2O���ʴ�Ϊ��Al(OH)3+OH-�TAlO2-+2H2O��
��7��ȼ�ŵ�þ�����ڶ�����̼�м���ȼ����������þ��̼����Ӧ�Ļ�ѧ����ʽΪCO2+2Mg![]() 2MgO+C���ʴ�Ϊ��CO2+2Mg
2MgO+C���ʴ�Ϊ��CO2+2Mg![]() 2MgO+C��
2MgO+C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�ء�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ��![]() ��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��1��AΪ_______ ��(д��Ԫ�ط��ţ���ͬ)�������Ų�ʽ��________________________��
BΪ_______�������Ų�ʽ��________________________��
CΪ_______���۵����Ų�ʽ��________________________��
DΪ_______�������Ų�ͼ��________________________��
EΪ_______��ԭ�ӽṹʾ��ͼ��________________________��
��2��A��B��C��D����Ԫ�ص縺���ɴ�С��˳��Ϊ________________________��
��3��A��B��C��D����Ԫ�ص�һ��������С�����˳��Ϊ_____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС��Ϊ��֤Ũ�����ܽ�NO������NO2����ϡ���������NO�������ͼװ�ý���ʵ�飨�г���������ȥ�������������ϣ���NO��NaOH��Һ����Ӧ��NO2��NaOH��Һ�ܷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ2NO2+2NaOHNaNO3+NaNO2+H2O����
��ѡҩƷ��Ũ���ᡢ3mol��L-1ϡ���ᡢ����ˮ��Ũ���ᡢNaOH��Һ��CO2���塣�ش��������⡣

��1�����Ӻ�װ�ã��μ�Ũ����֮ǰ�IJ��������ǣ�____________������ҩƷ����װ�â��еĵ��ɼк�ͨ��CO2һ��ʱ�䣬�رյ��ɼУ���װ�â��е���ĩ�����뵹�õ���ƿ�ڡ�ͨ��CO2��Ŀ����____________��
��2��������������Ϊ70%Ũ���ᣨ�ܶ�Ϊ1.42g��cm-3������250mL3mol��L-1��ϡ���ᣬ����Ͳ���ձ�����ͷ�ι��⣬��Ҫ�õ��IJ���������____________��____________��
��3��װ�â��з�����Ӧ�Ļ�ѧ����ʽΪ____________��
��4��װ�â��в���������ͨ��ʢ��ˮ��ϴ��ƿʱ������Ӧ�Ļ�ѧ����ʽΪ____________��
��5������ʵ����ƣ�װ�â���ʢ�ŵ��Լ���____________��
��6����ʵ��ʱӦ�����к������ŷŵ������У�װ�â���ʢ�ŵ�ҩƷ��____________��
��7����֤Ũ�����ܽ�NO������NO2����ϡ���������NO��ʵ��������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ�����ʵ����ʵ���Ũ��Ϊ1 mol/L���ǣ� ��
A. ��40gNaOH��������1Lˮ��
B. ��22.4 L�Ȼ�����������ˮ���1L��Һ
C. ��1L 10mol/L��Ũ������9Lˮ���
D. ��10g NaOH�����ܽ���ˮ�����250 mL��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д�����з�Ӧ�Ļ�ѧ����ʽ����ָ����Ӧ���͡�
��1���屽���Ʊ�_________________________________________________��_____________����
��2���üױ��Ʊ�TNT _____________________________________________��_____________����
��3���ɱ�ϩ�Ʊ��۱�ϩ____________________________________________��_____________����
��4�����������������Ƶ��Ҵ���Һ����______________________________��_____________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС����ʵ���ҴӺ���������ȡ�Ⲣ�Ʊ�KI���塣��ش���������
(1)��ˮ��Һ����ȡ�����ѡ�õ��Լ���____________��(�����)
A���ƾ� B��CCl4 C����ϩ D��ֱ������
(2)KI������Ʊ���ʵ��װ����ͼ��

ʵ�鲽������
i������0.5mol��L1��KOH��Һ��
i��������ƿ�м���12.7g����I2��250mL 0.5mol��L1��KOH��Һ������������ȫ�ܽ⡣
����ͨ����Һ©����Ӧ�����Һ�еμ��������ᣬ��ַ�Ӧ��HCOOH������ΪCO2������KOH��Һ��pH��9~10����������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ������KI��Ʒ8.3g����ش��������⣺
������0.5mol��L1 KOH��Һʱ�����в���������õ���ҺŨ��ƫ�ߵ���_____(�����)��
A�������Ϸֱ����������ȵ�ֽƬ�����KOH����
B��KOH������Ʒ�л���K2O2
C�������õĹ�������ձ����ܽ�δ����ȴֱ��ת��������ƿ
D��δϴ���ձ���������ֱ��������ƿ�м�ˮ����
E������ʱ���ӿ̶���
�ڲ��袢��I2��KOH��Һ��Ӧ���ɵ���������ͻ�ԭ��������ʵ���֮��Ϊ1��5����д����������Ļ�ѧʽ��____________��
�۲��袣������Һ�еμ���������ʱ�������___________��(�a����b����a��b��)
��ʵ���У�����HCOOH����������ԭ��Ӧ�����ӷ���ʽΪ____________________��
��ʵ����KI�IJ���Ϊ________________%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£�ijһ�ܱ������������������п��淴ӦA��g����3B��g��![]() 2C��g�����÷�Ӧ���е�һ���Ⱥ�ﵽ�˻�ѧƽ�⣬�û�ѧƽ��ı�־�ǣ� ��
2C��g�����÷�Ӧ���е�һ���Ⱥ�ﵽ�˻�ѧƽ�⣬�û�ѧƽ��ı�־�ǣ� ��
A.C���ʵ��������ʺͷֽ��������
B.������������ܶȲ�����ʱ����ı�
C.��λʱ��������amol����A��ͬʱ����3amol����B
D.A��B��C�ķ�����֮��Ϊ1��3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ϊȼ�ϵ�ȼ�ϵ�أ���������Ч�ʸߵ��ص㣬���ⰱ���������ߣ���Һ����������������棬�Ǻܺõ���Դ���塣NH3��O2ȼ�ϵ�صĽṹ��ͼ��ʾ������˵����ȷ����( )
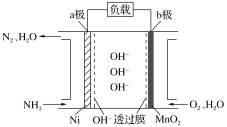
A.a��Ϊ��ص�����
B.�����ĵ缫��ӦʽΪ2NH3��6e����6OH��=N2��6H2O
C.������1 mol N2ʱ����·��ͨ���ĵ��ӵ����ʵ���Ϊ3 mol
D.���·�ĵ�������Ϊ��a������b��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£�pH��11�İ�ˮ��NaOH��Һ�ֱ��ˮϡ��100������Һ��pH����Һ����仯��������ͼ��ʾ������ͼ���жϴ�����ǣ� ��

A. aֵһ������9
B. ��ΪNaOH��Һϡ��ʱ��Һ��pH�仯����
C. ��ȫ�к�ϡ����ͬ�����������Һ��������ͬŨ�ȵ�ϡH2SO4�����V(NaOH)<V(��ˮ)
D. ϡ�ͺ�ˮ��ˮ�ĵ���̶ȱ�NaOH��Һ��ˮ�ĵ���̶ȴ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com