
����Ŀ���� X��Y��Z ����Ԫ�أ�X ���л��������бغ���Ԫ�أ� Y �ǵؿ��ﺬ������Ԫ�أ�Z ���� �������Ԫ�ء�X��Y��Z ����Ԫ����ɵ��л��� M �ܱ����Ը�������������� N��Ϊ�˲ⶨ�л��� M �Ľṹ��������ʵ�飺
���� 4.6 g �л��� M ��ȫȼ�գ�������� 0.2mol CO2 �� 5.4 g ˮ�� ���������Ǽ���л��� M���õ���ͼ 1 ��ʾ������ͼ�� ���ú˴Ź����Ǵ����л��� M���õ���ͼ 2 ��ʾͼ�ס�

�Իش��������⣺
��1��M �Ľṹ��ʽ��___________________��N �к��еĹ����ŵĽṹ��ʽΪ_____________��
��2��д�� M ��ͭ�������Ҽ���������������������Ӧ�Ļ�ѧ����ʽ___________________��
��3��д�� M �� N ��Ũ H2SO4���������·�����Ӧ�Ļ�ѧ����ʽ___________________��
���𰸡� CH3CH2OH ��COOH 2CH3CH2OH��O2![]() 2CH3CHO��2H2O CH3COOH��CH3 CH2OH
2CH3CHO��2H2O CH3COOH��CH3 CH2OH![]() CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
��������X��Y��Z ����Ԫ�أ�X���л��������бغ���Ԫ�أ���XΪ̼Ԫ�أ�Y�ǵؿ��ﺬ������Ԫ�أ���YΪ��Ԫ�أ�Z �����������Ԫ�أ���ZΪ��Ԫ�ء�
�������Ǽ���л��� M���õ���ͼ 1 ��ʾ������ͼ������Է�������Ϊ46��ͨ�����㣬������̼�����ʵ���Ϊ0.2mol��ˮ�����ʵ���Ϊ![]() =0.3mol�������л����к���ԭ�ӵ����ʵ���=
=0.3mol�������л����к���ԭ�ӵ����ʵ���=![]() =0.1mol������̼������ԭ�Ӹ�����Ϊ2:6:1��������Է���������������ѧʽΪC2H6O�����ݺ˴Ź�������������������ԭ�ӣ�����Ϊ3:2:1������M�Ľṹ��ʽΪ��CH3CH2OH��M �ܱ����Ը�������������� N��NΪCH3COOH����1��M �Ľṹ��ʽ��CH3CH2OH��N ΪCH3COOH�����еĹ����ŵĽṹ��ʽΪ-COOH����2��CH3CH2OH��ͭ�������Ҽ���������������������Ӧ����CH3CHO����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH��O2
=0.1mol������̼������ԭ�Ӹ�����Ϊ2:6:1��������Է���������������ѧʽΪC2H6O�����ݺ˴Ź�������������������ԭ�ӣ�����Ϊ3:2:1������M�Ľṹ��ʽΪ��CH3CH2OH��M �ܱ����Ը�������������� N��NΪCH3COOH����1��M �Ľṹ��ʽ��CH3CH2OH��N ΪCH3COOH�����еĹ����ŵĽṹ��ʽΪ-COOH����2��CH3CH2OH��ͭ�������Ҽ���������������������Ӧ����CH3CHO����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O����3��CH3CH2OH �� CH3COOH��Ũ H2SO4���������·�����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH��CH3 CH2OH
2CH3CHO��2H2O����3��CH3CH2OH �� CH3COOH��Ũ H2SO4���������·�����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH��CH3 CH2OH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1) ______mol CO2�к�����ԭ������1.806��1024��H2O���Ӻ��е���ԭ������ͬ��
(2)0.4 mol SiH4����������ԭ������________g HCl����������ԭ������ȡ�
(3)��״���µļ����һ����̼�Ļ������8.96 L��������Ϊ7.60 g�����������м�������Ϊ____________��һ����̼������Ϊ___________��
(4)�����ʵ���O2�ͳ���(O3)��������֮��Ϊ______________����O2��O3������ȣ�����ԭ����֮��Ϊ_____________��
(5)��MgCl2��KCl��Na2SO4�������ʵĻ��Һ�У���֪���к�Cl��1.5 mol��K����Na����1.5 mol��Mg2��Ϊ0.5 mol����SO42-������Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���20.0 g 14%��NaCl��Һ��30.0 g 24%��NaCl��Һ��ϣ���Ϻ�õ��ܶ�Ϊ 1.17 g��cm��3����Һ������㣺
��1����Ϻ����Һ��NaCl�����������Ƕ��٣�___________
��2����Ϻ����Һ�����ʵ���Ũ��Ϊ���٣�_________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ԭ��Ӧ3S+6KOH=K2SO3+2K2S+3H2O�У��������뱻��ԭ����ԭ����֮��Ϊ�� ��
A.1��1
B.2��1
C.1��2
D.3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л���A����ʽΪCxHyOz��15g A��ȫȼ������22g CO2��9gH2O��
(1)���л�������ʽ��___________________��
(2)��A��һ����ɫ�ߴ�ǿ���ݼ�����ζ�����壬���л�ԭ�ԣ�����ṹ��ʽ��_________��
(3)A��ֻ��һ�ֹ����ţ���A��Na2CO3���������ų����ʹ��ܷ���������Ӧ����A�Ľṹ��ʽΪ___________________��
(4)A��ֻ��һ�ֹ����ţ���A���ӷ���ˮ����ζ��Һ�壬�ܷ���ˮ�ⷴӦ������ṹ��ʽΪ___________________��
(5)��A����ӽṹ�к���6��̼ԭ�ӣ����ж�Ԫ����ȩ�����ʣ�����ṹ��ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����ijNa2CO3��Һ�м���ϡ���ᣬ��Һ�к�̼�������ʵ����������գ�����ҺpH�仯�IJ��������ͼ��ʾ������˵������ȷ����
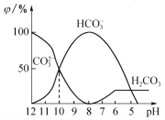
A. Na2CO3��Һ��c(Na+)=c(CO32-��+ c(HCO3-��+ c(H2CO3��
B. pH=8ʱ����Һ��c(Cl-)=c(Na+)
C. pH =7ʱ����Һ�е�c(Na+)>c(Cl-)>c(HCO3-)>c(H+)=c(OH-)
D. 25��ʱ��CO32-+H2O![]() HCO3-+OH-��Kh=10��10mol��L��1
HCO3-+OH-��Kh=10��10mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I���м����������ǻ����Ȼ����ֻ��ţ���������������γɵĻ������У�д�������������ʵ����ʵĽṹ��ʽ��
��1����ʹʯ����Һ��ɫ����______�֣�
��2���������������������������ͭ����������ԭ��Ӧ����______
��3����ͬ��������������Ʒ�Ӧ�����������ε���______��
II���л���A���������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
ʵ�鲽�� | ���ͻ�ʵ����� |
(1)��ȡA 9.0 g������ʹ�������������ܶ�����ͬ������H2��45���� | (1)A����Է�������Ϊ��________. |
(2)����9.0 g A��������O2�г��ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4 g��13.2 g | (2)A�ķ���ʽΪ��________. |
(3)��ȡA 9.0 g����������NaHCO3��ĩ��Ӧ������2.24 L CO2(��״��)���������������Ʒ�Ӧ������2.24 L H2(��״��)�� | (3)�ýṹ��ʽ��ʾA�к��еĹ����ţ�________��________. |
(4)A�ĺ˴Ź�����������ͼ��
| (4)A�к���________����ԭ�ӣ� |
(5)����������A�Ľṹ��ʽ________. | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������У��ֱ��������ƣ��ֺ�NaOH��Һ��Ӧ����
A. ��ˮ�ƾ� B. ���� C. �� D. 75%�ľƾ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com