
����Ŀ����Ԫ����һ����Ҫ�ķǽ���Ԫ�أ����γɶ��ֻ�����Իش������й����⣺
��1������֪4CO(g)��2NO2(g) ![]() 4CO2(g)��N2(g) ��H����1 200 kJ��mol��1���ڸ÷�Ӧ���ı�ijһ��Ӧ����������֪�¶�T2>T1������ͼ����ȷ����_______(�����)
4CO2(g)��N2(g) ��H����1 200 kJ��mol��1���ڸ÷�Ӧ���ı�ijһ��Ӧ����������֪�¶�T2>T1������ͼ����ȷ����_______(�����)

����֪CO��H2O��һ�������¿��Է�����Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g)����H= �� Q kJ��mol��1 ��820 ��ʱ�ڼס������������ܱ������У���ʼʱ�����±�����Ͷ�ϣ�����һ��ʱ���ﵽƽ��״̬��������CO��ת����Ϊ40%����÷�Ӧ��ƽ�ⳣ��Ϊ____________�����������յ�����Ϊ________________��
CO2(g)��H2(g)����H= �� Q kJ��mol��1 ��820 ��ʱ�ڼס������������ܱ������У���ʼʱ�����±�����Ͷ�ϣ�����һ��ʱ���ﵽƽ��״̬��������CO��ת����Ϊ40%����÷�Ӧ��ƽ�ⳣ��Ϊ____________�����������յ�����Ϊ________________��
![]()
��2���¿���Ϊ�����������ȼ�ϣ���������N2O4��Ӧ����N2��ˮ��������֪��
�� N2 (g) +2O2 (g) = N2O4 (l) ��H =��19.5 kJ��mol��1
�� N2H4(l)+O2(g) = N2(g) +2H2O(g) ��H =��534.2 kJ��mol��1
д������������������Ӧ���Ȼ�ѧ��ʽ______________��
��3�����ĵ�����(HN3)����ɫҺ�壬����������������������25 mL 0.1mol��L��1 NaOH��Һ�м���0. 2mol��L��1 HN3����Һ���μӹ����е�pHֵ�ı仯���ߣ���Һ���ʱ������仯���Բ��ƣ���ͼ��

�ٸ���ͼ��д��HN3�ĵ��뷽��ʽ:_________��
������˵����ȷ����_________________������ţ�
A��������֪Ũ�ȵ�NaOH��Һ�ζ�HN3��Һ���ⶨHN3��Ũ��ʱӦ�ü�����ָʾ��
B�������£���0.2 mol��L��1 HN3����Һ�м�ˮϡ�ͣ���![]() ����
����
C���ֱ��к�pH��Ϊ4��HN3��Һ��HCl��Һ������0.1 mol��L��1 NaOH��Һ�������ͬ
D��D��ʱ��Һ������Ũ�ȴ������¹�ϵ��2c(H+) + c(HN3) =c(N3��) + 2c(OH��)
���𰸡��� ![]() 0.12Q kJ 2N2H4(l) + N2O4 (l) = 3N2 (g) + 4H2O (g) ����=-1048.9 kJ��mol-1 HN3
0.12Q kJ 2N2H4(l) + N2O4 (l) = 3N2 (g) + 4H2O (g) ����=-1048.9 kJ��mol-1 HN3![]() H++N3�D BD
H++N3�D BD
��������
��1���ٸ���Ӱ�컯ѧ��Ӧ���ʵ����أ��¶ȡ�Ũ�ȡ�ѹǿ��������ȷ����ѧ��Ӧ��ƽ���õ���ʱ�䣬����Ӱ�컯ѧƽ���ƶ������أ��¶ȡ�Ũ�ȡ�ѹǿ��ȷ����ѧƽ���и������ı仯�����
�ڸ��ݻ�ѧƽ������ʽ��ʽ�Լ���ѧ��Ӧ��CO��ת����Ϊ40%���м��㼴�ɣ�
��2�����ݸ�˹���ɣ��������Ȼ�ѧ����ʽ����д����
��3��������ͼ���֪�������������ᣬ�ݴ���д���뷽��ʽ������
��A���ζ��յ�ʱ����Һ�����Ա�Ϊ���ԣ��ݴ�ѡ��ָʾ����
B������Kֻ���¶��йؽ��з�����
C������������ڵ�����з�����
D�����������غ�͵���غ��жϡ�
��1���ټף������¶ȣ���ѧƽ�������ƶ��������淴Ӧ���ʻ�Ѹ�������뿪ԭ�������ʵ㣬�ʼ״����ң������¶ȣ���ѧ��Ӧ������������T2ʱ�ȴﵽ��ѧƽ��״̬�����һ�ѧƽ�������ƶ�������������ת���ʼ�С��������ȷ���������ڷ�Ӧ��4CO(g)��2NO2(g) 4CO2(g)��N2(g)��T���䣬����ѹǿ��ƽ�������ƶ���һ����̼������������С���ʱ�����ѡ����
����֪����CO��ת����Ϊ40%�����У�
CO(g)��H2O(g)![]() CO2(g)��H2(g)
CO2(g)��H2(g)
��ʼ����0.1 0.1 0 0
�仯����0.04 0.04 0.04 0.04
ƽ������0.1-0.04 0.1-0.04 0.04 0.04
���������ݻ�ΪVL����K=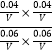 =
=![]() ��
��
�ں��º��������£��Һͼ�Ϊ��Чƽ����ƽ�ⳣ����ͬ����
CO(g)��H2O(g)![]() CO2(g)��H2(g)
CO2(g)��H2(g)
��ʼ����0 0 0.2 0.2
�仯����x x x x
ƽ������x x 0.2- x 0.2- x
��K=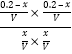 =
=![]() �����x=0.12�������������յ�����Ϊ0.12Q kJ��
�����x=0.12�������������յ�����Ϊ0.12Q kJ��
��2���� N2 (g) +2O2 (g) = N2O4 (l) ��H1=��19.5 kJ��mol��1
�� N2H4(l)+O2(g) = N2(g)+2H2O(g) ��H2 =��534.2 kJ��mol��1
���ݸ�˹�����ڡ�2-���õ��º�������������Ӧ���Ȼ�ѧ��ʽΪ2N2H4(l) + N2O4 (l) = 3N2 (g) + 4H2O (g) ����=-1048.9 kJ��mol-1��
��3��������ͼ���֪�������������ᣬ������ȫ���룬�ÿ���ţ��ʵ��뷽��ʽΪ��HN3![]() H++N3-���ʴ�Ϊ��HN3
H++N3-���ʴ�Ϊ��HN3![]() H++N3-��
H++N3-��
��A���ζ��յ�ʱ������Ϊ����������Һ�������������ˮ���Լ��ԣ���Һ�����Ա�Ϊ���ԣ�Ӧѡ�÷�̪��Һ��ָʾ������A������
B�������£���0.2 molL-1HN3����Һ�м�ˮϡ�ͣ�ˮ�����ӻ��������䣬����ƽ�ⳣ�����䣬���У�Kw=c(H+)��c(OH-)��Ka=![]() ��Ka=
��Ka= = Kw��img src="http://thumb.zyjl.cn/questionBank/Upload/2019/05/03/08/785509a5/SYS201905030836124054790767_DA/SYS201905030836124054790767_DA.007.png" width="85" height="45" style="-aw-left-pos:0pt; -aw-rel-hpos:column; -aw-rel-vpos:paragraph; -aw-top-pos:0pt; -aw-wrap-type:inline" />��Ka���䣬Kw���䣬��
= Kw��img src="http://thumb.zyjl.cn/questionBank/Upload/2019/05/03/08/785509a5/SYS201905030836124054790767_DA/SYS201905030836124054790767_DA.007.png" width="85" height="45" style="-aw-left-pos:0pt; -aw-rel-hpos:column; -aw-rel-vpos:paragraph; -aw-top-pos:0pt; -aw-wrap-type:inline" />��Ka���䣬Kw���䣬��![]() ��������B��ȷ��
��������B��ȷ��
C��pHֵ��ȵ���������������Ѿ��������������Ũ����ͬ����ʱ��������������Һ��ͬ���������ڵ����������ᣬ����������������ӻ���Ҫ�������ƽ����кͣ��ʵ��������ĵ��������Ƶ������C����
D��D���ɵ����ʵ���Ũ�ȵ�NaN3��HN3��ɵĻ��Һ�����������غ㣺2c��Na+��=c��N3-��+c��HN3�����͵���غ�c��Na+��+c��H+��=c��N3-��+c��OH-�����ɵ�2c(H+) + c(HN3) =c(N3��) + 2c(OH��)����D��ȷ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��T��ʱ��ˮ�����ӻ�ΪKw�����¶��½�a mol/LһԪ��HA��Һ��b mol/LһԪ��BOH��Һ�������ϣ�����Ϻ���Һ�����ԣ�������˵��һ����ȷ���ǣ� ��
A. ���Һ�У�c(H+) = ![]() B. ���Һ��pH=7
B. ���Һ��pH=7
C. ���Һ�У�c(B+) = c(A��) + c(OH��) D. a = b
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳ���ϩ����Ҫ��Ӧ��6H2(g)+2CO2(g)![]() CH2=CH2(g)+4H2O(g) ��H<0��ͼ��L(L1��L2)�����ɷֱ����ѹǿ���¶ȡ�����˵����ȷ����
CH2=CH2(g)+4H2O(g) ��H<0��ͼ��L(L1��L2)�����ɷֱ����ѹǿ���¶ȡ�����˵����ȷ����

A. L1<L2
B. X����ѹǿ
C. M��N�����Ӧ��ƽ�ⳣ����ͬ
D. M�������Ӧ����v��С��N����淴Ӧ����v��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ������ ��
A. ������FeCl3��Һ�����ˮ���ƽ��壬���ӷ���ʽ��Fe3++3H2O![]() Fe(OH)3 ��+3H+
Fe(OH)3 ��+3H+
B. Ϊȷ��ij��H2A��ǿ�ỹ�����ᣬ�ɲ�NaHA��Һ��pH����pH>7����H2A�������pH<7����H2A��ǿ��
C. һ��������ʹ��ѧƽ��������Ӧ�����ƶ�����Ӧ���ת���ʲ�һ������
D. ����ʱ����ij���淴Ӧ��ѧƽ�ⳣ��Kֵ��С��������÷�Ӧ����H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��250Cʱ����c(CH3COOH)+c(CH3COO��)=0.1mol��L-1��һ����ᡢ�����ƻ����Һ����Һ��c(CH3COOH)��c(CH3COO��)��pH �Ĺ�ϵ��ͼ7 ��ʾ�������й���Һ������Ũ�ȹ�ϵ��������ȷ����

A. pH="5." 5 ����Һ�У� c(CH3COOH)>c(CH3COO��)>c(H��)>c(OH��)
B. W ������ʾ����Һ�У� c(Na��)+c(H��)= c(CH3COOH)+c(OH��)
C. pH =" 3." 5 ����Һ�У� c(Na��) +c(H��) -c(OH��) +c(CH3COOH)=0.1mol��L-1
D. ��W ������ʾ��1.0L ��Һ��ͨ��0.05mol HCl ����(��Һ����仯�ɺ���)�� c(H��)= c(CH3COOH)+c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ��������������ȷ���ǣ� ��
A��1.8 g��NH4+ �����к��еĵ�����Ϊ0.1NA
B��1mol Na2O2 �����к���������Ϊ4NA
C����״���£�2.24L CCl4�����Ĺ��ۼ���ΪO.4NA
D�����³�ѹ�£�92g NO2��N2O4�Ļ�����庬�е�ԭ����Ϊ6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£�xA��yB![]() zC�ķ�Ӧ�ﵽƽ�⡣
zC�ķ�Ӧ�ﵽƽ�⡣
��1����֪A��B��C�������壬�ڼ�ѹ��ƽ�����淴Ӧ�����ƶ�����x��y��z֮��Ĺ�ϵ��__________________��
��2����֪C�����壬��x��y��z��������ѹǿʱ�����ƽ�ⷢ���ƶ�����ƽ��һ����_____________�ƶ���
��3����֪B��C�����壬�������������䣬����A�����ʵ���ʱ��ƽ�ⲻ�����ƶ�����A��_____________̬���ʡ�
��4�������Ⱥ�C�������������٣�������Ӧ��_________��������������������������Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ǵ�������Ҫ��Ⱦ��֮һ������ԭSO2������������SO2����Ⱦ�������Եõ���ҵԭ��S��ȼú��������Ļ��շ�ӦΪ��2CO(g)+SO2(g) ![]() 2CO2(g)+S(l) ��H��
2CO2(g)+S(l) ��H��
(1)��֪��2CO(g)+O2(g)===2CO2(g) ��H1=��566.0kJ��mol��1
S(l)+O2(g)===SO2(g) ��H2=��296.8 kJ��mol��1
����Ļ��շ�Ӧ����H=___________ kJ��mol��1��
(2)����������ͬ��������ͬʱ����Ļ��շ�Ӧ��SO2��ת�����淴Ӧ�¶ȵı仯��ͼ��ʾ��260��ʱ��___________������La2O3������NiO������TiO2��)�Ĵ�Ч����ߡ�La2O3��NiO������������ʹSO2��ת���ʴﵽ�ܸߣ������Ǽ۸����أ�ѡ��La2O3����Ҫ�ŵ���___________��

(3)һ�������£����ں�ѹ�ܱ������з�����Ļ��շ�Ӧ��SO2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����P1��P2��P3��P4�ɴ�С��˳��Ϊ___________��ij�¶��£����ں����ܱ������У���ʼʱc(CO)=2 a mol��L��1��c(SO2)= a mol��L��1��SO2��ƽ��ת����Ϊ80%������¶��·�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ___________��
(4)ijʵ��С��Ϊ̽���������١��¶ȶԸ÷�Ӧ��Ӱ�죬��La2O3���������ֱ������ֲ�ͬ������������ͬ�¶��½���ʵ�顣ʵ������ʾ����260��ʱ��SO2��ת���������������������С����ԭ����___________����380��ʱ��SO2��ת�������������������������ԭ����___________��
(5)��ҵ�ϳ���Na2SO3��Һ���������е�SO2��������ͨ��1.0 mol��L��1��N2SO3��Һ������ҺpHԼΪ6ʱ������SO2�����������½���ʱ��Һ��c(HSO3��)c�U(SO32��)=___________��(��֪H2SO3��Ka1=1.5��10-2��Ka2=1.0��10-7)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Բ��ϲ�ҵ��21���������չ�ĸ߿Ƽ�֧����ҵ֮һ����Ϊ��Ϣ��ҵ�ͻ��繤ҵ����Ҫ�������ܲ��ϣ����Բ��Ϲ㷺Ӧ���ڵ�����Ϣ�����¼���������̼������Ҫ�����Ʊ����������壬��ҵ�������̿�(��Ҫ�ɷ�ΪMnO2)�ͻ�����(��Ҫ�ɷ�ΪFeS2)Ϊ��Ҫԭ���Ʊ�̼���̵���Ҫ�����������£�

��֪�����ֽ������ӳ�����pH���±���
Fe2�� | Fe3�� | Cu2�� | Mn2�� | |
��ʼ������pH | 7.5 | 3.2 | 5.2 | 8.8 |
��ȫ������pH | 9.2 | 3.7 | 7.8 | 10.4 |
�ش��������⣺
(1)Ϊ������ܽ�������ԭ�ϵĽ���Ч�ʣ���ȡ�Ĵ�ʩ����������________��
A������ B���ʵ������¶�
C����ĥ��ʯ D����������������ˮ
(2)�ܽ���������Ҫ����������ΪFe3����Mn2����SO42-����д����Ҫ��Ӧ�����ӷ���ʽ��___________����ȡ�����Һ�к�������Fe2����Cu2����Ca2�������ڼ���ʯ�ҵ�����Һ��pH�Ӷ�ʹ��Ԫ�ر���ȫ����ǰ���������������̿�Ŀ����______________________������ʯ�ҵ�����ҺpH�ķ�ΧΪ____________________��
(3)���������Ŀ���dz�ȥ��Һ�е�Cu2����Ca2�������ʣ���������Ҫ��________(�ѧʽ)��CaF2���������Һ��c(F��)Ϊ0.01mol��L��1������Һ�в���c(Ca2��)Ϊ________mol��L��1[��֪��Ksp(CaF2)��1.46��10��10]��
(4)������Ϊ����������ʹ��NaF��������Ⱦ��������(NH4)2CO3����NaF��������(NH4)2CO3����NaF��ȱ����______________________________________��
(5)��ͼΪ������������������̽����ʵ�Ӱ�죬����ͼ����Ϣ�����������������Ӧ������________%���ҡ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com