
°ĺŐ‚ńŅ°Ņ““»©(CH3CHO) «”–ĽķļŌ≥…÷–Ķń∂ĢŐľ ‘ľŃ£¨ «ļŌ≥…““ňŠ°Ę““īľ°Ę““ňŠ““ű•°ĘŇ©“©DDTĶ»Ķń‘≠ŃŌ°£ĽōīūŌ¬Ń–ő Ő‚:
(1)Andrea Dasic Ķ»ŐŠ≥Ų‘ŕĹū ŰīŖĽĮľŃM◊ų”√Ō¬“‘N2Oő™—űĽĮľŃŅ…“‘—űĽĮ““Ō©…ķ≥…““»©°£īŖĽĮŐŚŌĶ—űĽĮĽĻ‘≠—≠Ľ∑»ÁÕľňý ĺ°£(őÔ÷ ”Ž—ű‘≠◊”ĶńĹŠļŌѶ”√OAĪŪ ĺ)

—ű‘≠◊””ŽN…ķ≥…NOĶńĹŠļŌѶOA(N)= 167.4kJ°§mol-1,—ű‘≠◊””Ž““Ō©…ķ≥…““»©ĶńĹŠļŌѶOA(C2 H4)=473 kJmol-1,‘ÚŅ…”√◊ųł√∑ī”¶īŖĽĮľŃĶńĹū ŰM”Ž—ű‘≠◊”ĶńĹŠļŌѶOA(M)Ķń÷Ķ”¶¬ķ◊„:_______, Ļ”√īŖĽĮľŃĽŠ Ļł√∑ī”¶ĶńĽÓĽĮń‹____(ŐÓ°į‘Ųīů°ĪĽÚ°įľű–°°Ī)°£
(2)“—÷™CO(g)°ĘCH4(g)°ĘCH3CHO(l)Ķń»ľ…’»»∑÷Īūő™283.0 kJmol-1°Ę890.31 kJ°§ mol-1°Ę1167.9 kJmol-1,‘Ú““»©Ķń∑÷Ĺ‚∑ī”¶CH3CHO(l) ![]() CH4(g)+CO(g)Ķń H =________°£
CH4(g)+CO(g)Ķń H =________°£
(3)“—÷™:‘ŕļ¨”–…ŔŃŅI2Ķń»‹“ļ÷–,∑ī”¶CH3CHO(aq) ![]() CH4 (g)+CO(g)∑÷ŃĹ≤ĹĹÝ––:
CH4 (g)+CO(g)∑÷ŃĹ≤ĹĹÝ––:
ĶŕI≤Ĺ∑ī”¶ő™CH3CHO(aq) +I2(aq)°ķCH3I(l) + HI(aq) +CO(g)(¬ż∑ī”¶),ĶŕII≤Ĺő™Ņž∑ī”¶°£
ĘŔ«Ž–ī≥ŲĶŕII≤Ĺ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ:__________°£
Ęŕ‘ŲīůI2ĶńŇ®∂»______(ŐÓ°įń‹"ĽÚ°į≤Ľń‹")√ųŌ‘‘Ųīů◊‹∑ī”¶Ķń∆ĹĺýňŔ¬ ,ņŪ”…ő™_________°£
(4)““»©Ņ…“‘”ŽĪ•ļÕĶńNaHSO3»‹“ļ∑Ę…ķ∑ī”¶…ķ≥…ňģ»‹–‘Ķń![]() -Ű«ĽýĽ«ňŠń∆:CH3CHO+ NaHSO3
-Ű«ĽýĽ«ňŠń∆:CH3CHO+ NaHSO3![]() CH3CH(OH)SO3Na(
CH3CH(OH)SO3Na(![]() -Ű«ĽýĽ«ňŠő™“◊»‹”ŕňģĶń«ŅňŠ)°£∑ī”¶īÔĶĹ∆Ĺļ‚ļů,»Ű∆šňŻŐűľĢ≤ĽĪš,ŌÚ∑ī”¶ŐŚŌĶ÷–ľ”»Ž◊„ŃŅ—őňŠ£¨∆Ĺļ‚Ĺę___ (ŐÓ°į ’żŌÚ°Ī°įńśŌÚ°ĪĽÚ°į≤Ľ")“∆∂Į°£
-Ű«ĽýĽ«ňŠő™“◊»‹”ŕňģĶń«ŅňŠ)°£∑ī”¶īÔĶĹ∆Ĺļ‚ļů,»Ű∆šňŻŐűľĢ≤ĽĪš,ŌÚ∑ī”¶ŐŚŌĶ÷–ľ”»Ž◊„ŃŅ—őňŠ£¨∆Ĺļ‚Ĺę___ (ŐÓ°į ’żŌÚ°Ī°įńśŌÚ°ĪĽÚ°į≤Ľ")“∆∂Į°£
(5)‘ŕ100~120 °„C°ĘPdCl2 ®C CuCl2īŖĽĮľŃīś‘ŕŌ¬,““Ō©Ņ…“‘”ŽO2∑ī”¶…ķ≥…““»©: 2CH2=CH2(g) +O2(g) ![]() 2CH3CHO(g)°£ T°„C Ī£¨ŌÚ2 LĶńļ„»›√‹Ī’»›∆ų÷–Õ®»Ž3 mol CH2=CH2(g)ļÕ3 mol O2(g),∑Ę…ķ…Ō Ų∑ī”¶,∑ī”¶ł’ļ√īÔĶĹ∆Ĺļ‚◊īŐ¨ļůŐŚŌĶ—Ļ«ŅĪšő™≥ű ľ—Ļ«ŅĶń5/6£¨‘ÚCH2=CH2(g)Ķń∆Ĺļ‚◊™ĽĮ¬ ő™____ (ĹŠĻŻĪ£ŃŰ3őĽ”––ß ż◊÷),T °„C Īł√∑ī”¶Ķń∆Ĺļ‚≥£ żKő™________°£
2CH3CHO(g)°£ T°„C Ī£¨ŌÚ2 LĶńļ„»›√‹Ī’»›∆ų÷–Õ®»Ž3 mol CH2=CH2(g)ļÕ3 mol O2(g),∑Ę…ķ…Ō Ų∑ī”¶,∑ī”¶ł’ļ√īÔĶĹ∆Ĺļ‚◊īŐ¨ļůŐŚŌĶ—Ļ«ŅĪšő™≥ű ľ—Ļ«ŅĶń5/6£¨‘ÚCH2=CH2(g)Ķń∆Ĺļ‚◊™ĽĮ¬ ő™____ (ĹŠĻŻĪ£ŃŰ3őĽ”––ß ż◊÷),T °„C Īł√∑ī”¶Ķń∆Ĺļ‚≥£ żKő™________°£
°ĺīūįł°Ņ167.4kJ°§mol-1£ľOA(M) £ľ473 kJmol-1 ľű–° +5.41 kJ°§mol1 CH3I(l) + HI(aq) °ķ CH4(g) + I2(aq) ń‹ ◊‹∑ī”¶Ķń∆ĹĺýňŔ¬ ”…¬ż∑ī”¶ĺŲ∂®£¨I2 «¬ż∑ī”¶Ķń∑ī”¶őÔ£¨‘ŲīůĶńI2ĶńŇ®∂»£¨¬ż∑ī”¶ňŔ¬ ‘Ųīů£¨◊‹∑ī”¶Ķń∆ĹĺýňŔ¬ ‘Ųīů ńśŌÚ 66.7% 4
°ĺĹ‚őŲ°Ņ
ĘŇ”…īŖĽĮľŃĶń◊ų”√ĽķņŪĹŠļŌÕľ ĺ–ŇŌĘ÷™£¨ĶĪ—ű‘≠◊””ŽīŖĽĮľŃĶńĹŠļŌѶī¶”ŕ÷–ľš÷Ķ Ī£¨īň∑ī”¶Ņ…∑Ę…ķ£¨īŖĽĮľŃĽŠĹĶĶÕ∑ī”¶ĶńĽÓĽĮń‹°£
Ę∆∑÷Īū–ī≥Ų»ľ…’»»Ķń»»∑ī”¶∑Ĺ≥Ő Ĺ£¨‘Ŕ”√Ķŕ3łŲĶń»»∑ī”¶∑Ĺ≥Ő Ĺľű»•Ķŕ1łŲļÕĶŕ2łŲĶń»»∑ī”¶∑Ĺ≥Ő Ĺ°£
Ę«ĘŔłýĺ›ĶŕI≤Ĺ∑ī”¶ļÕīŖĽĮľŃĶń∑ī”¶‘≠ņŪ£¨◊‹∑ī”¶ľű»•ĶŕI≤Ĺ∑ī”¶Ķ√ĶĹĶŕII≤Ĺ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ£ĽĘŕ◊‹∑ī”¶ňŔ¬ ”…¬ż∑ī”¶ňŔ¬ ĺŲ∂®£¨I2 «¬ż∑ī”¶Ķń∑ī”¶őÔ£¨“Úīň‘ŲīůI2ĶńŇ®∂»ń‹√ųŌ‘‘Ųīů◊‹∑ī”¶Ķń∆ĹĺýňŔ¬ °£
Ę»ŌÚ∑ī”¶ŐŚŌĶ÷–ľ”»Ž◊„ŃŅ—őňŠ£¨—őňŠŅ…“‘”ŽNaHSO3∑ī”¶£¨ Ļ∑ī”¶őÔNaHSO3ĶńŇ®∂»ľű–°£¨∆Ĺļ‚ńśŌÚ“∆∂Į°£
Ę…Ĺ®ŃĘ»ż∂ő Ĺ£¨łýĺ›—Ļ«Ņ÷ģĪ»Ķ»”ŕőÔ÷ ĶńŃŅ÷ģĪ»ĹÝ––ľ∆ň„£¨‘Ŕň„◊™ĽĮ¬ ļÕ∆Ĺļ‚≥£ ż°£
ĘŇ—ű‘≠◊””ŽN…ķ≥…NOĶńĹŠļŌѶOA(N) = 167.4 kJ°§mol1£¨—ű‘≠◊””Ž““Ō©…ķ≥…““»©ĶńĹŠļŌѶOA(C2H4)=473 kJ°§mol1£¨”…īŖĽĮľŃĶń◊ų”√ĽķņŪĹŠļŌÕľ ĺ–ŇŌĘ÷™£¨ĶĪ—ű‘≠◊””ŽīŖĽĮľŃĶńĹŠļŌѶī¶”ŕ÷–ľš÷Ķ Ī£¨īň∑ī”¶Ņ…∑Ę…ķ£¨“ÚīňŅ…”√◊ųł√∑ī”¶īŖĽĮľŃĶńĹū ŰM”Ž—ű‘≠◊”ĶńĹŠļŌѶOA(M)Ķń÷Ķ”¶¬ķ◊„167.4kJ°§mol-1£ľOA(M) £ľ473 kJmol-1£¨īŖĽĮľŃĽŠĹĶĶÕ∑ī”¶ĶńĽÓĽĮń‹£ĽĻ īūįłő™£ļ167.4 kJ°§mol1£ľOA(M) £ľ473 kJ°§mol1£Ľľű–°°£
Ę∆“—÷™CO(g)°ĘCH4(g)°ĘCH3CHO(l)Ķń»ľ…’»»∑÷Īūő™283.0 kJ°§mol1°Ę890.31 kJ°§mol1°Ę1 167.9 kJ°§mol1£¨∑÷Īū–ī≥Ų»ľ…’»»Ķń»»∑ī”¶∑Ĺ≥Ő Ĺ£¨‘Ŕ”√Ķŕ3łŲĶń»»∑ī”¶∑Ĺ≥Ő Ĺľű»•Ķŕ1łŲļÕĶŕ2łŲĶń»»∑ī”¶∑Ĺ≥Ő Ĺ£¨‘Ú““»©Ķń∑÷Ĺ‚∑ī”¶CH3CHO(l) ![]() CH4(g)+CO(g)Ķń H =1167.9 kJ°§mol1 (283.0 kJ°§mol1) (890.31 kJ°§mol1) = +5.41 kJ°§mol1£ĽĻ īūįłő™£ļ+5.41 kJ°§mol1°£
CH4(g)+CO(g)Ķń H =1167.9 kJ°§mol1 (283.0 kJ°§mol1) (890.31 kJ°§mol1) = +5.41 kJ°§mol1£ĽĻ īūįłő™£ļ+5.41 kJ°§mol1°£
Ę«ĘŔłýĺ›ĶŕI≤Ĺ∑ī”¶ļÕīŖĽĮľŃĶń∑ī”¶‘≠ņŪ£¨◊‹∑ī”¶ľű»•ĶŕI≤Ĺ∑ī”¶Ķ√ĶĹĶŕII≤Ĺ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő ĹCH3I(l) + HI(aq) °ķ CH4(g) + I2(aq)£ĽĻ īūįłő™£ļCH3I(l) + HI(aq) °ķ CH4(g) + I2(aq)°£
Ęŕ◊‹∑ī”¶ňŔ¬ ”…¬ż∑ī”¶ňŔ¬ ĺŲ∂®£¨I2 «¬ż∑ī”¶Ķń∑ī”¶őÔ£¨“Úīň‘ŲīůI2ĶńŇ®∂»ń‹√ųŌ‘‘Ųīů◊‹∑ī”¶Ķń∆ĹĺýňŔ¬ £ĽĻ īūįłő™£ļń‹£Ľ◊‹∑ī”¶Ķń∆ĹĺýňŔ¬ ”…¬ż∑ī”¶ĺŲ∂®£¨I2 «¬ż∑ī”¶Ķń∑ī”¶őÔ£¨‘ŲīůĶńI2ĶńŇ®∂»£¨¬ż∑ī”¶ňŔ¬ ‘Ųīů£¨◊‹∑ī”¶Ķń∆ĹĺýňŔ¬ ‘Ųīů°£
Ę»∑ī”¶īÔĶĹ∆Ĺļ‚ļů£¨»Ű∆šňŻŐűľĢ≤ĽĪš£¨ŌÚ∑ī”¶ŐŚŌĶ÷–ľ”»Ž◊„ŃŅ—őňŠ£¨—őňŠŅ…“‘”ŽNaHSO3∑ī”¶£¨ Ļ∑ī”¶őÔNaHSO3ĶńŇ®∂»ľű–°£¨∆Ĺļ‚ńśŌÚ“∆∂Į£ĽĻ īūįłő™£ļńśŌÚ°£
Ę…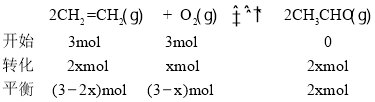
![]() £¨Ĺ‚Ķ√x = 1mol£¨‘ÚCH2=CH2Ķń∆Ĺļ‚◊™ĽĮ¬ ő™
£¨Ĺ‚Ķ√x = 1mol£¨‘ÚCH2=CH2Ķń∆Ĺļ‚◊™ĽĮ¬ ő™![]() £¨T °„C Īł√∑ī”¶Ķń∆Ĺļ‚≥£ ż
£¨T °„C Īł√∑ī”¶Ķń∆Ĺļ‚≥£ ż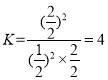 £ĽĻ īūįłő™£ļ66.7%£Ľ4°£
£ĽĻ īūįłő™£ļ66.7%£Ľ4°£


 ŐžŐžŌÚ…Ō“ĽĪĺļ√ĺŪŌĶŃ–īūįł
ŐžŐžŌÚ…Ō“ĽĪĺļ√ĺŪŌĶŃ–īūįł –°—ß…ķ10∑÷÷””¶”√Ő‚ŌĶŃ–īūįł
–°—ß…ķ10∑÷÷””¶”√Ő‚ŌĶŃ–īūįł
| ńÍľ∂ | łŖ÷–Ņő≥Ő | ńÍľ∂ | ≥ű÷–Ņő≥Ő |
| łŖ“Ľ | łŖ“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű“Ľ | ≥ű“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ∂Ģ | łŖ∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű∂Ģ | ≥ű∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ»ż | łŖ»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű»ż | ≥ű»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° |
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ£®1£©Ō÷”–Ō¬Ń–őÔ÷ ĘŔNaClĺßŐŚĘŕ“ļŐ¨SO2 ĘŘīŅī◊ňŠ Ę‹ŃÚňŠĪĶĘ›Õ≠ Ęřĺ∆ĺę(C2H5OH) ĘŖ»ŘĽĮĶńKNO3 ĘŗNaOH»‹“ļ ĘŠįĪňģ «Ž”√“‘…ŌőÔ÷ Ķń–ÚļŇĽōīūŌ¬Ń–ő Ő‚£ļ
Ű”ŕ«ŅĶÁĹ‚÷ Ķń «________£Ľ Ű”ŕ»űĶÁĹ‚÷ Ķń «_______£Ľ‘ŕ…Ō Ų◊īŐ¨Ō¬ń‹ĶľĶÁĶńőÔ÷ «_________°£
£®2£©ĽĮ—ß∆Ĺļ‚“∆∂Į‘≠ņŪÕ¨—ý“≤ ”√”ŕ∆šňŁ∆Ĺļ‚£¨“—÷™‘ŕįĪňģ÷–īś‘ŕŌ¬Ń–∆Ĺļ‚£ļNH3°§H2O ![]() NH4£ę£ęOH£≠
NH4£ę£ęOH£≠
ĘŔŌÚįĪňģ÷–ľ”»ŽNH4ClĻŐŐŚ Ī£¨∆Ĺļ‚____________“∆∂Į£¨(ŐÓ°įŌÚ”“°ĪĽÚ°įŌÚ◊ů°Ī)£¨c(OH£≠)____(ŐÓ°į‘Ųīů°ĪĽÚ°įľű–°°Ī£¨Ō¬Õ¨)°£
ĘŕŌÚįĪňģ÷–ľ”»ŽMgCl2ĻŐŐŚ Ī£¨∆Ĺļ‚_____“∆∂Į£¨(ŐÓ°įŌÚ”“°ĪĽÚ°įŌÚ◊ů°Ī)£¨ c(NH4£ę)____(ŐÓ°į‘Ųīů°ĪĽÚ°įľű–°°Ī)
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅŌ¬ĪŪő™‘™ňō÷‹∆ŕĪŪĶń“Ľ≤Ņ∑÷£¨«Ž≤ő’’‘™ňōĘŔ£≠ĘŠ‘ŕĪŪ÷–ĶńőĽ÷√£¨”√ĽĮ—ß”√”ÔĽōīūŌ¬Ń–ő Ő‚£ļ
◊Ś ÷‹∆ŕ | IA | 0 | ||||||
1 | ĘŔ | ĘÚA | ĘůA | ĘŰA | ĘűA | ĘŲA | ĘųA | |
2 | Ęŕ | ĘŘ | Ę‹ | Ę› | ||||
3 | Ęř | ĘŖ | Ęŗ | ĘŠ | ||||
(1)‘≠◊”įŽĺ∂◊Ó–°Ķń‘™ňō «___(ŐÓ‘™ňō√Ż≥∆)£¨–ī≥ŲĘŗĶń‘≠◊”ĹŠĻĻ ĺ“‚Õľ____________°£
(2)◊ÓłŖľŘ—űĽĮőÔ∂‘”¶ĶńňģĽĮőÔ÷–£¨ľÓ–‘◊Ó«ŅĶń «___(”√ĽĮ—ß ĹĽōīū£¨Ō¬Õ¨)£¨ňŠ–‘◊Ó«ŅĶń «____°£
(3)ĘŔ”ŽĘ›–ő≥…ĶńĽĮļŌőÔ÷–£¨ĽĮ—ßľŁņŗ–Õő™____°£
(4)ĘŖ”ŽĘŠ–ő≥…ĶńĽĮļŌőÔĶńĶÁ◊” Ĺő™__£¨Ęŕ”ŽĘ‹–ő≥…Ķń‘≠◊”łŲ żĪ»ő™1:2ĶńĽĮļŌőÔĶńĹŠĻĻ Ĺő™__°£
(5)ĘŔ”ŽĘŕ–ő≥…Ķń“Ľ÷÷ŐĢ£¨ĺŖ”–∆Ĺ√ś’żŃýĪŖ–őĹŠĻĻ£¨Ōŗ∂‘∑÷◊”÷ ŃŅő™78£¨–ī≥ŲīňŐĢ∑Ę…ķ»°īķ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ(»ő–ī“ĽłŲľīŅ…)£ļ_°£
(6)ő™ŐĹĺŅ‘™ňōĘŕļÕĘŗĶń∑«Ĺū Ű–‘«Ņ»ű£¨ń≥Õ¨—ß…Ťľ∆Ńň»ÁÕľňý ĺĶń◊į÷√ĹÝ–– Ķ—ť(ľ–≥÷“«∆ų“—¬‘»•£¨◊į÷√∆Ý√‹–‘Ńľļ√)°£«ŽĽōīū£ļ
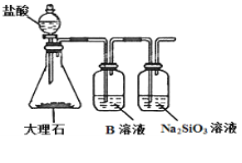
ĘŔ»‹“ļBő™______£¨B»‹“ļĶń◊ų”√ «______°£
Ęŕ»ŰŅīĶĹ_______Ō÷Ōů£¨ľīŅ…÷§√ųňŠ–‘_______(”√ĽĮ—ß ĹĽōīū)£¨‘Ú∑«Ĺū Ű–‘______(”√‘™ňō∑ŻļŇĽōīū)°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņī”ļ£īÝ÷–ŐŠ»°Ķ‚£¨Ņ…ĺ≠Ļż“‘Ō¬ Ķ—ť≤Ĺ÷ŤÕÍ≥…°£Ō¬Ń–”–ĻōňĶ∑®’ż»∑Ķń «
![]()
A. ◊∆…’Ļż≥Ő÷– Ļ”√Ķń≤£Ńß“«∆ų”–ĺ∆ĺęĶ∆°Ę…’Ī≠°Ę≤£ŃßįŰ
B. —űĽĮĻż≥Ő÷–∑Ę…ķ∑ī”¶ĶńņŽ◊”∑Ĺ≥Ő Ĺő™ 2I-£ęH2O2 =I2£ę2OH-
C. ľž—ťĶ‚Ķ•÷ Ī£¨Ņ…—°”√ĶŪ∑ŘĶ‚ĽĮľō ‘÷Ĺ£¨»Ű ‘÷ĹĪšņ∂ňĶ√ųļ£īÝ÷–ļ¨”–Ķ‚Ķ•÷
D. ∑÷“ļ Ī£¨Ō»īÚŅ™ĽÓ»Ż∑Ň≥ŲŌ¬≤„“ļŐŚ£¨‘ŔĻōĪ’ĽÓ»Żī”…ŌŅŕĶĻ≥Ų…Ō≤„“ļŐŚ
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ‘ŕ0.l mol°§L-1ĶńCH3COOH»‹“ļ÷–īś‘ŕĶÁņŽ∆Ĺļ‚£ļCH3COOH![]() CH3COO£≠£ęH£ę∂‘”ŕł√∆Ĺļ‚£¨Ō¬Ń––ū Ų’ż»∑Ķń «
CH3COO£≠£ęH£ę∂‘”ŕł√∆Ĺļ‚£¨Ō¬Ń––ū Ų’ż»∑Ķń «
A. ľ”»Ž…ŔŃŅCH3COONH4ĻŐŐŚ£¨∆Ĺļ‚≤Ľ“∆∂Į£¨c(H+)≤ĽĪš
B. ľ”»Ž…ŔŃŅNaOHĻŐŐŚ£¨∆Ĺļ‚ŌÚ’żŌÚ“∆∂Į£¨»‹“ļ÷–c(H+)ľű–°
C. ľ”ňģ£¨∆Ĺļ‚ŌÚ’żŌÚ“∆∂Į£¨c(CH3COOH)/ c(CH3COO£≠)‘Ųīů
D. Õ®»Ž…ŔŃŅ HCl∆ÝŐŚ£¨∆Ĺļ‚ńśŌÚ“∆∂Į£¨»‹“ļ÷–c(H+)ľű…Ŕ
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ“©őÔ÷–ľšŐŚ(G)‘ŕ”–Ľķ÷∆“©Ļ§“Ķ÷–Ķń“Ľ÷÷ļŌ≥…∑Ĺ∑®»ÁŌ¬:
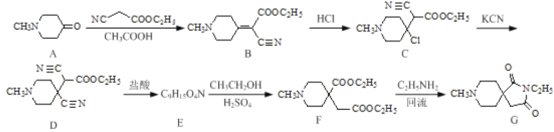
ĽōīūŌ¬Ń–ő Ő‚:
(1)ĽĮļŌőÔDļÕG÷–ļ¨—űĻŔń‹ÕŇĶń√Ż≥∆∑÷Īūő™___________°Ę_________°£
(2)”…B°ķCĶń∑ī”¶ņŗ–Õő™_____ ;–ī≥ŲC°ķ D∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ:________°£
(3)ĽĮļŌőÔEĶńĹŠĻĻľÚ Ĺő™________°£
(4)∑ī”¶F°ķGĶńŃŪ“Ľ÷÷…ķ≥…őÔ «___________°£
(5)–ī≥ŲÕ¨ Ī¬ķ◊„Ō¬Ń–ŐűľĢĶńBĶńÕ¨∑÷“žĻĻŐŚĶńĹŠĻĻľÚ Ĺ:_______°£
ĘŔń‹”Ž–¬÷∆Cu(OH)2ľ”»»ŐűľĢŌ¬∑ī”¶…ķ≥…◊©ļž…ę≥ŃĶŪ£¨ňģĹ‚≤ķőÔ÷ģ“Ľń‹”ŽFeCl3»‹“ļ∑Ę…ķŌ‘…ę∑ī”¶:
ĘŕļňīŇĻ≤’Ů«‚∆◊ő™ňń◊ť∑Ś,∑Ś√śĽżĪ»ő™1:2:4:9;
ĘŘ∑÷◊”÷–ļ¨”–įĪĽý°£
(6)“—÷™:RCN![]() RCH2NH2«Ž…Ťľ∆“‘HOOCCH2COOHļÕCH3CH2Clő™‘≠ŃŌ÷∆Īł
RCH2NH2«Ž…Ťľ∆“‘HOOCCH2COOHļÕCH3CH2Clő™‘≠ŃŌ÷∆Īł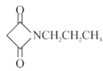 ĶńļŌ≥…¬∑ŌŖ:_________(őřĽķ ‘ľŃ»ő”√)°£
ĶńļŌ≥…¬∑ŌŖ:_________(őřĽķ ‘ľŃ»ő”√)°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅÕÍ≥…Ō¬Ń–ŐÓŅ’
£®1£©įĪ∑÷◊”ĶńĶÁ◊” Ĺ «____°£ĽĮļŌőÔ![]() įīŌĶÕ≥√Ł√Ż∑®Ķń√Ż≥∆ő™________°£
įīŌĶÕ≥√Ł√Ż∑®Ķń√Ż≥∆ő™________°£
£®2£©–ī≥ŲńĺŐŅ”ŽŇ®ŌűňŠľ”»»∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ£ļ___________°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņń≥—–ĺŅ–°◊ť“‘AgZSMő™īŖĽĮľŃ£¨‘ŕ»›Ľżő™1LĶń»›∆ų÷–£¨ŌŗÕ¨ ĪľšŌ¬≤‚Ķ√0.1molNO◊™ĽĮő™N2Ķń◊™ĽĮ¬ ňśő¬∂»ĪšĽĮ»ÁÕľňý ĺ[őřCO Ī∑ī”¶ő™2NO(g)![]() N2(g)+O2(g)£Ľ”–CO Ī∑ī”¶ő™2CO(g)+2NO(g)
N2(g)+O2(g)£Ľ”–CO Ī∑ī”¶ő™2CO(g)+2NO(g)![]() 2CO2(g)+N2(g)]°£Ō¬Ń–ňĶ∑®īŪőůĶń «£® £©
2CO2(g)+N2(g)]°£Ō¬Ń–ňĶ∑®īŪőůĶń «£® £©
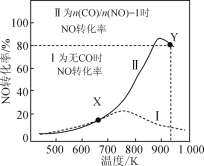
A.∑ī”¶2NO![]() N2+O2Ķń¶§H£ĺ0
N2+O2Ķń¶§H£ĺ0
B.īÔ∆Ĺļ‚ļů£¨∆šňŻŐűľĢ≤ĽĪš£¨ Ļ![]() £ĺ1£¨CO◊™ĽĮ¬ Ō¬ĹĶ
£ĺ1£¨CO◊™ĽĮ¬ Ō¬ĹĶ
C.XĶ„Ņ…“‘Õ®ĻżłŁĽĽłŖ–ßīŖĽĮľŃŐŠłŖNOĶń∑ī”¶ňŔ¬
D.YĶ„‘ŔÕ®»ŽCO°ĘN2łų0.01mol£¨īň Īv(CO£¨’ż)£ľv(CO£¨ńś)
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņń≥–°◊ťÕ¨—ßņŻ”√»ÁÕľňý ĺ◊į÷√ŐĹĺŅ∂Ģ—űĽĮŃÚ∆ÝŐŚĶń–‘÷ £ģ
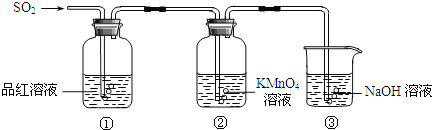
«ŽĽōīū£ļ
£®1£© Ķ—ť “”√Õ≠ļÕŇ®ŃÚňŠĻ≤»»÷∆»°∂Ģ—űĽĮŃÚ£¨∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺ « £ģ
£®2£©ĶĪĻŘ≤žĶĹĘŕ÷–»‹“ļ—’…ęĪš«≥ Ī£¨ĘŔ÷–ĶńŌ÷Ōů « £ģ
£®3£©◊į÷√ĘŘĶń◊ų”√ « £ģ
£®4£©ĘŔ°ĘĘŕ÷–ĶńŌ÷ŌůňĶ√ų∂Ģ—űĽĮŃÚĺŖ”–Ķń–‘÷ « £ģ
≤ťŅīīūįłļÕĹ‚őŲ>>
Ļķľ —ß–£”Ň—° - Ń∑Ōį≤ŠŃ–ĪŪ - ‘Ő‚Ń–ĪŪ
ļĢĪĪ °Ľ•Ń™ÕÝő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®∆ĹŐ® | ÕÝ…Ō”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | ĶÁ–Ň’©∆≠ĺŔĪ®◊®«Ý | …śņķ ∑–ťőř÷ų“Ś”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | …ś∆ů«÷»®ĺŔĪ®◊®«Ý
ő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®ĶÁĽį£ļ027-86699610 ĺŔĪ®” Ōš£ļ58377363@163.com