
����Ŀ��ij�о�С�鰴����·�ߺϳ���ζ����˹���� ��
��

��֪���ٷ��㻯����A�ܷ���������Ӧ���˴Ź���������ʾ��5�ֲ�ͬ��ѧ��������ԭ�ӣ�
��
��RCN![]() RCOOH
RCOOH
��
�ش��������⣺
(1)F�Ľṹ��ʽ��_________________________________________��
(2)����˵����ȷ����________��
A.������A�Ĺ��������ǻ�
B.������B�ɷ�����ȥ��Ӧ
C.������C�ܷ����ӳɷ�Ӧ
D.������D�ɷ����Ӿ۷�Ӧ
(3)д����˹����������NaOHˮ��Һ��ַ�Ӧ�Ļ�ѧ����ʽ��_________��
(4)д��ͬʱ�������������� D��ͬ���칹��Ľṹ��ʽ��_________��
�������ֻ�ѧ������ͬ����ԭ�ӣ��ں��������������ʡ�
(5)���������ϳ�·�ߣ����һ���ɼ�ȩΪ��ʼԭ���Ʊ���������ĺϳ�·��________��
���𰸡� BC
BC 
 ��
��

��������
������AΪ�����廯�����A�к��б���������A�ķ���ʽ���Լ�A�ܷ���������Ӧ��A�к���ȩ�����˴Ź���������5�ֲ�ͬ��ѧ��������ԭ�ӣ����ݱ������������A�Ľṹ��ʽΪ![]() ��������Ϣ�ڣ��Ƴ�B�Ľṹ��ʽΪ
��������Ϣ�ڣ��Ƴ�B�Ľṹ��ʽΪ ��������Ϣ�ۣ��Ƴ�C�Ľṹ��ʽΪ
��������Ϣ�ۣ��Ƴ�C�Ľṹ��ʽΪ ��������Ϣ�ܣ���NH2ȡ���ǻ���λ�ã���D�Ľṹ��ʽΪ
��������Ϣ�ܣ���NH2ȡ���ǻ���λ�ã���D�Ľṹ��ʽΪ �����ݷ�Ӧ����Ƴ�F�Ľṹ��ʽΪ
�����ݷ�Ӧ����Ƴ�F�Ľṹ��ʽΪ ���ݴ˷�����
���ݴ˷�����
��1����������������F�Ľṹ��ʽΪ ��
��
��2��A������A�Ľṹ��ʽ��A�к��й�������ȩ������A����
B������B�Ľṹ��ʽ���ǻ�����̼ԭ�ӵ����ڵ�λ������H�����ܷ�����ȥ��Ӧ����B��ȷ��
C��C�к��б������ܷ����ӳɷ�Ӧ����C˵����ȷ��
D��������D�в���̼̼˫�������������ܷ����Ӿ۷�Ӧ����D����
��ѡBC��
��3�����ݰ�˹����Ľṹ��ʽ��1mol��˹�����к���1mol�Ȼ���1mol������1mol�ļ������1mol��˹�����������3molNaOH���仯ѧ����ʽΪ +3NaOH
+3NaOH ![]()
 ��CH3OH��H2O��
��CH3OH��H2O��
��4�������ֲ�ͬ��ѧ��������ԭ�ӣ�˵���ǶԳƽṹ�����л��������ԣ����л����в����Ȼ�����NH2�����ǻ�������D�Ľṹ��ʽ����ͬ���칹���к��У�NO2������Ҫ��ͬ���칹���� ��
�� ��
��
��5����������Ľṹ��ʽΪH2NCH2COOH��ԭ��Ϊ��ȩ��Ŀ������ǰ������ᣬ������һ��̼ԭ�ӣ���ȩ����HCN�����ӳɷ�Ӧ������HOCH2CN��������Һ����HOCH2COOH��Ȼ����һ�������£���NH3����H2NCH2COOH�����ϳ�·��Ϊ ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������1mol��ѧ������Ҫ����������:
��ѧ�� | N-N | O=O | N��N | N-H |
����kJ��mol��1 | a | 500 | 942 | 391 |
�£�N2H4�����йط�Ӧ�����仯��ͼ��ʾ������˵����ȷ���ǣ� ��

A. ��ͼ��ʾ:1molN2H4��1molO2��Ӧ����1molN2��2molH2O��Ӧ����H=��2752kJ��mol��1
B. a=154
C. N2(g)+2H2O(g)=N2H4(g)+O2(g) ��H=+2752kJ��mol��1
D. ����2molH2O(g)����ʱ���ų�534kJ��mol��1������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽������A����ɺ����ʣ����ʵ�鲢�������ת����

��֪��X�����ֻ�������ɣ�����Xͨ��Ʒ����Һ����Һ��ɫ������Xͨ������˫��ˮ�У�X��ȫ����������ֻ�õ�һ��ǿ�ᣬ��ϡ�͵�1000mL�������Һ��PH=1������Һ2�еμ�KSCN��Һ����Һ��Ѫ��ɫ����ش�
��1������A�Ļ�ѧʽ______________��
��2��д����Ӧ�ٵĻ�ѧ����ʽ____________��
��3��д����Ӧ��������A�����ӷ���ʽ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ӵ�������ֵΪNA������˵������ȷ����
A.���³�ѹ�£�2 g D2O �к�������ΪNA
B.1mol����ϩ��![]() �������к���̼̼˫���ĸ���Ϊ4 NA
�������к���̼̼˫���ĸ���Ϊ4 NA
C.0.5mol�ǻ�������������Ϊ4.5NA
D.28g��ϩ�ͱ�ϩ��C3H6���Ļ�����庬�е�̼ԭ����ĿΪ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾ����װ�D���һ����ⱥ��ʳ��ˮ�����ⶨ������������ ����ͼ������������Ե�ʵ��װ�á�

��1����ѡ��������ʱ�����ӿڵ�˳���ǣ�����ӿڵĴ�����ĸ����a�� �� �� ��b�� �� �� ��______________
��2��֤����Cl2���ɵ�ʵ��������____________��
��3��ʵ��ʱ��װ���е����缫�ӵ�Դ��____________����ʯī�缫�ķ�ӦʽΪ____________��
��4��װ��E��ȡ�������ʱ��Ӧ���е�ʵ�������____________����ʵ�������װ��E�Ķ�����������ɱ�״����Ϊ5.60mL��������Һ�����ǡ��Ϊ50.0mL������Һ��OH-��Ũ��Ϊ________mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.ij��Ӧ�����Ϊ4 L�ĺ����ܱ������н��У� ��0-3�����ڸ����ʵ����ı仯�����ͼ��ʾ��A��B��C��Ϊ���壬��A��������ɫ����

(1)�÷�Ӧ�Ļ�ѧ����ʽΪ____________________
(2)��Ӧ��ʼ��2����ʱ��B��ƽ����Ӧ����Ϊ__________��A��ת����Ϊ________________________
(3)��˵���÷�Ӧ�Ѵﵽƽ��״̬����______________
a.�������������ɫ���ֲ���
b.�����ڻ��������ܶȱ��ֲ���
c.v����B��= 2v����C��
d.�����ڻ�������ƽ����Է����������ٸı�
II.��ͼ������ȼ�ϵ�ع���ʾ��ͼ��
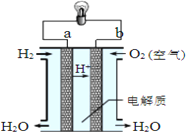
�õ�ع���ʱ�����ӵ�����______��_____���a����b������ÿ����1 mol H2O�������ϵ�·��ͨ���ĵ�����Ϊ__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�кܶ���Ҫ�Ļ���ԭ�϶���Դ��ʯ�ͻ�������ͼ�еı�����ϩ���л���A�ȣ�����A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ش��������⣺

(1)A�Ľṹ��ʽΪ________________����ϩ���й����ŵ�����Ϊ__________________________________________
(2)д�����з�Ӧ�ķ�Ӧ������___________________����________________________
(3)����˵����ȷ������________��
A.�������л���ŨHNO3��H2SO4�����䵹�뵽NaOH��Һ�У����ã���Һ
B.��ȥ���������е����ᣬ��NaOH��Һ����Һ
C.�۱�ϩ���ܹ�ʹ���Ը��������Һ��ɫ
D.�л���C���ϩ������ͬϵ��
(4)д�����з�Ӧ����ʽ��
��B��CH3CHO_________________________________________
�ܱ�ϩ�� + B����ϩ������_____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ˮ�ĵ���ƽ��������ͼ��ʾ���Իش��������⣺

(1)ͼ�����KW��Ĺ�ϵ��__________________��
(2)����A�㵽D�㣬�ɲ��õĴ�ʩ��________��
a������ b������������NaOH c������������NH4Cl
(3)E��Ӧ���¶��£���pH=9��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ��pH=7����NaOH��Һ��H2SO4��Һ�������Ϊ________��
(4)25��ʱ�����ΪVa��pH=a��ijһԪǿ����Һ�����ΪVb��pH=b��ijһԪǿ����Һ���Ȼ�Ϻ���Һ��pH=7����֪b=2a��Va��Vb�� ��a��ȡֵ��ΧΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣
��֪��Na2SO3+H2SO4��Na2SO4+SO2��+H2O

�ش��������⣺
��1��װ��A��ʢ��Ũ���������������____________��
��2��װ��B��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������____________��
��3��װ��C�б�����SO2��____________�ԣ�װ��D�б�����SO2��____________�ԣ�װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ____________��
��4��Fװ�õ�������____________��©����������____________��
��5��E�в�����ɫ�������ð�ɫ�����Ļ�ѧ�ɷ�Ϊ____________�����ţ���ͬ��,���ʵ��֤������ж�____________��
A.BaSO3 B.BaSO4 C.BaSO3��BaSO4
��6������úȼ�ղ�����������ֱ���ŷŵ������У���������Ҫ����������____________��
A.����ЧӦ B.���� C.�۳���Ⱦ D.ˮ�帻Ӫ����
��ҵ��Ϊʵ��ȼú����ͨ������ʯ��ʯ�õ���ʯ�ң�����ʯ��Ϊ���������������SO2��Ӧ�Ӷ�����̶������������������ϡ�д�����н���̶��Ļ�ѧ����ʽ��____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com